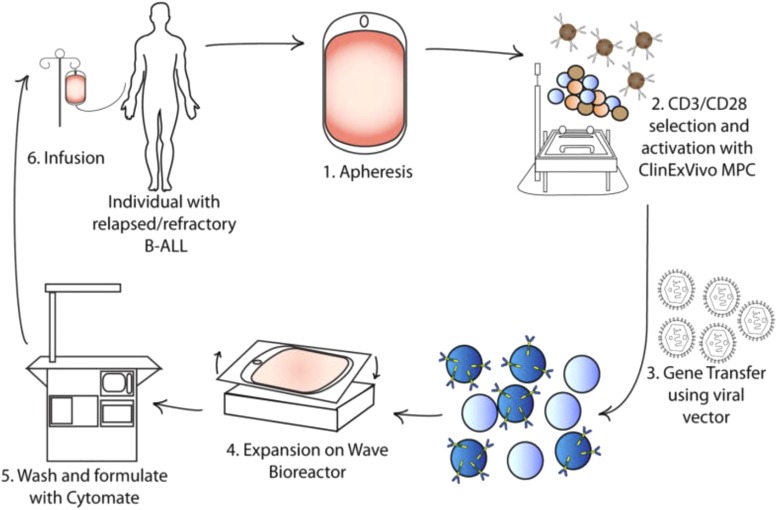
Fig. 6.
Production of CAR-T cells in a GMP facility. Production begins with (1) leukapheresis of the patient and is then followed by selection of T cells (2), and their activation, from the leukapheresis product by positive selection with a CD3 antibody ± anti-CD28 antibody. After a few days the activated T cells are incubated with retroviral supernatant to transfer the CAR gene (3). Expansion, washing, and formulation result in infusion back into the patient [from Davila et al. (2014a) with permission].