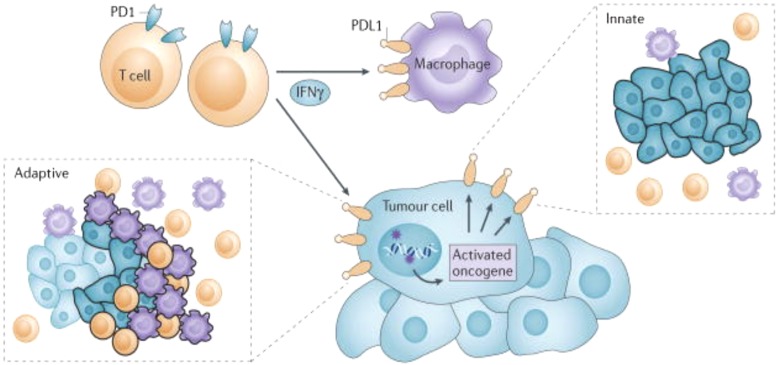
Fig. 7.
Constitutive broad (innate) expression of membranous programmed cell death 1 ligand 1 (PDL1) by tumor cells is thought to be driven by dysregulated signaling pathways such as PI3K-AKT, or chromosomal alterations and amplifications such as are found in Hodgkin lymphoma. In contrast, adaptive focal expression of PDL1 by tumor cells and macrophages occurs at the interface of tumor cell nests with immune infiltrates secreting pro-inflammatory factors such as interferon-γ (IFNγ; the “immune front”). The ligation of PDL1 with programmed cell death protein 1 (PD1) molecules will down-modulate T-cell function, essentially creating a negative feedback loop that dampens antitumour immunity. The innate and adaptive mechanisms for PDL1 induction are not mutually exclusive: constitutive oncogene-driven PDL1 expression may be further upregulated by inflammatory cytokines. In boxed insets, tumor cells are shown as blue, macrophages are purple, and T-cells are orange; black outlining of cells indicates PDL1 protein expression, such as would be demonstrated with immunohistochemistry [from Topalian et al. (2016) with permission].