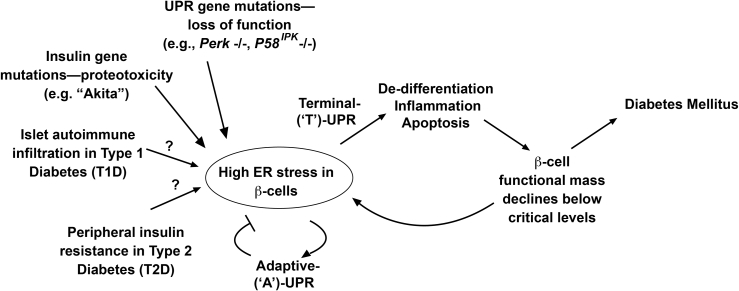
Figure 1.
‘A’- to ‘T’-UPR conversions may promote β-cell death in diabetes. During ER stress, adaptive UPR outputs maintain cellular homeostasis. If ER stress remains unmitigated, terminal UPR signaling drives destructive outputs culminating in β-cell apoptosis. Declining β-cell mass elevates workload/ER stress in remaining β-cells in vicious cycles promoting diabetes. These principles may be a unifying feature in monogenetic and polygenetic forms of diabetes (both rare and common). In various UPR gene mutations, loss of function of critical UPR components (e.g., PERK) dysregulates the pathway leading to infantile diabetes. Gain-of-function proteotoxicity accompanies mutant proinsulin expression in the MIDY syndrome caused by the (C96Y) Akita variant. In T1D, islet immune cell infiltration elevates ER stress in β-cells. Peripheral insulin resistance elevates β-cell overwork in T2D. It is conceivable that similar events that occur in the murine models drive β-cell degeneration in human T1D and T2D.