Figure 2.
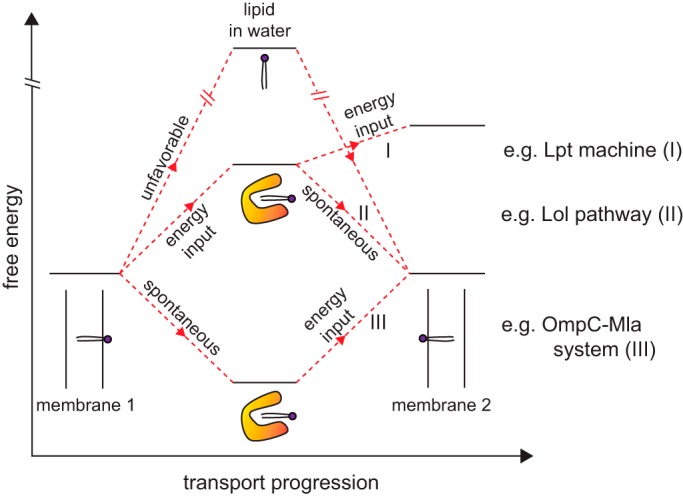
Possible free energy profiles for protein-mediated intermembrane lipid transport. Release of lipids from a membrane (donor) for unassisted diffusion across an aqueous environment to another membrane (recipient) is highly energetically disfavored. In known transport systems, lipid-binding proteins are central to shielding the acyl tails of lipid molecules upon release, giving rise to lipid–protein complexes with energy levels sufficiently close to those of lipids in the membrane environment; this renders lipid transport feasible with or without external energy input, e.g. derived from ATP hydrolysis. Shown here are three different scenarios with distinct energetic requirements at different stages: (I) energy is required to release the lipid molecule from donor membrane as well as to insert it into the recipient membrane, e.g. Lpt machine (Fig. 3); (II) energy is required to release the lipid molecule from the donor membrane only, whereas insertion into recipient membrane is spontaneous, e.g. Lol pathway (Fig. 3); and (III) release from donor membrane is spontaneous (due to high-affinity protein binding) but energy is then required to release the lipid molecule from protein for insertion into recipient membrane, e.g. OmpC–Mla pathway in the context of retrograde transport (Fig. 4). Donor and recipient membranes are labeled as membranes 1 and 2, respectively.
