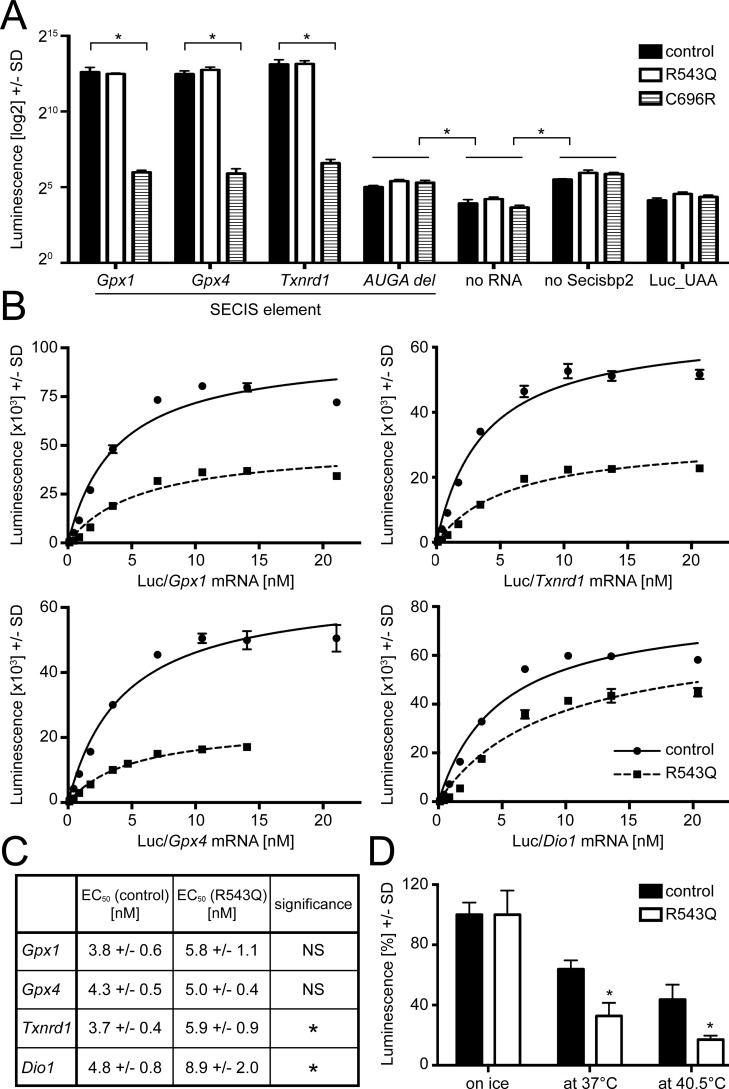
Figure 3.
In vitro functional analysis of recombinantly expressed mouse Secisbp2 R543Q and C696R. Reporter constructs containing a Sec-dependent luciferase cDNA and SECIS elements cloned into the 3′ UTR as given are in vitro translated, and the amount of luciferase produced was measured by an activity assay. A, Secisbp2 R543Q is functional, whereas C696R is not functional at all. Recombinant Secisbp2 at 160 nm was incubated with constructs containing murine Gpx1, Gpx4, and Txnrd1 SECIS elements, and luciferase activity was measured. A rat Gpx4 SECIS element lacking the AUGA sequence of the essential kink-turn served as negative control. Further negative controls were no mRNA added, no Secisbp2 protein added, and a luciferase carrying a UAA codon at the position of the UGA/Sec codon. A log2 scale is used to better appreciate the small increment of luciferase activity in the controls lacking Secisbp2 or the SECIS quartet over lack of mRNA or a definite stop codon indicating a small SECIS/Secisbp2-independent UGA read-through. B, kinetics of Secisbp2R543Q:SECIS binding compared with Secisbp2WT for several murine SECIS elements. The concentration of recombinant Secisbp2 was 80 nm, and 0.1–20 nm mRNA was used. The apparent KD of Secisbp2R543Q was significantly reduced for Dio1 and Gpx1 SECIS only, and the maximal amount of luciferase was decreased when using the mutant as compared with Secisbp2WT. C, thermal instability of Secisbp2R543Q in vitro. Recombinant Secisbp2 protein at 160 nm concentration was incubated for 30 min on ice, at 37 °C, and at 40.5 °C and then used for in vitro translation with the luciferase/Gpx4 SECIS construct. The luciferase activity values of the respective Secisbp2WT incubated on ice was defined as 100%. The experiment was done twice in triplicates. Error bars, S.D.