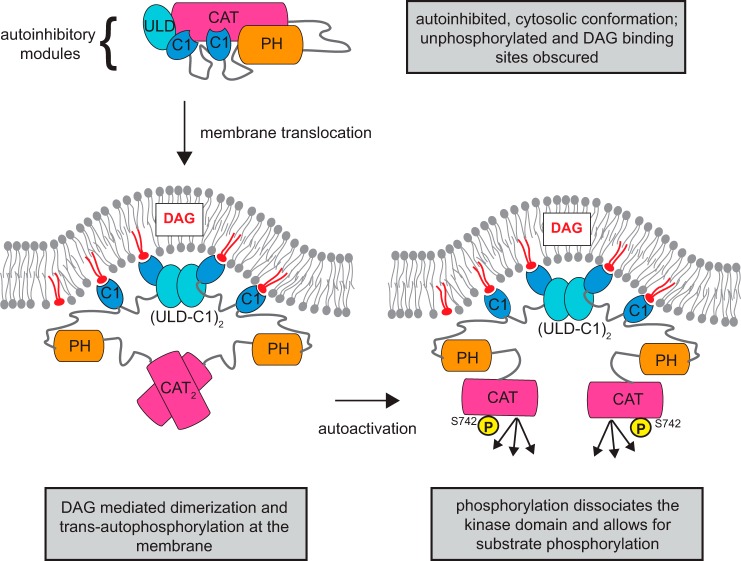
Figure 8.
Model of PKD autoactivation by ULD dimerization. PKD transitions from an autoinhibitory cytosolic conformation to an active DAG-bound conformation. Arrows indicate the sequence of events in this proposed activation mechanism. In the cytosol, the autoinhibitory conformation is maintained by the regulatory ULD, C1, and PH domains. This conformation is presumably unphosphorylated and monomeric, and the DAG-binding sites of the C1 domains are sequestered in this autoinhibitory conformation. When DAG is generated, PKD translocates to the membrane. The engagement of the C1a and C1b to DAG releases the autoinhibition of the kinase domain. Also, the increase in local concentration leads to dimerization of the ULD, which facilitates kinase domain dimerization and trans-autophosphorylation in the activation loop. Ultimately, phosphorylation of Ser742 breaks the dimeric arrangement of the kinase domains, which allows the kinase domains to engage and phosphorylate the substrates.