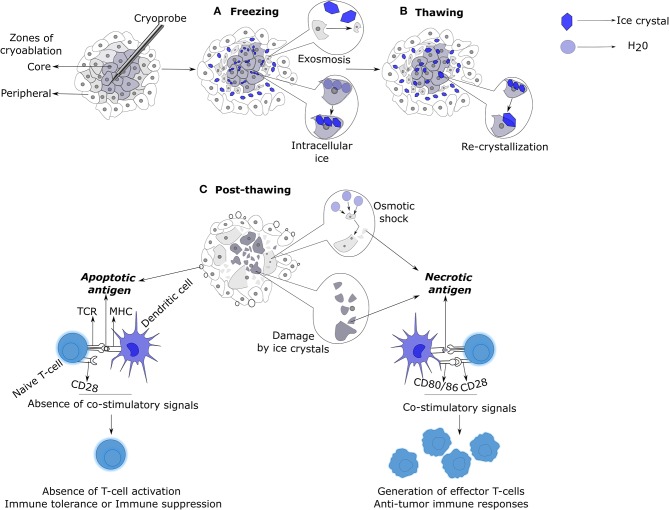
Figure 1.
Mechanisms of cell death and immunologic responses induced by cryoablation. (A) Cells in the core of the ablation zone are subjected to lethal temperatures at rapid freezing rates, resulting in the generation of extra and intracellular ice crystals. Cells adjacent to the core zone undergo moderate or low freezing rates. This permits the cells to lose intracellular water by exosmosis in response to the formation of extracellular ice crystals resulting in cellular dehydration and shrinkage. In contrast, cells in the core zone cannot undergo exosmosis due to rapid freezing rates and thus, form intracellular crystals. Both intra and extracellular ice crystals cause mechanical damage to the cells. (B) During the thawing phase, the small intracellular ice crystals, due to their thermodynamic instability, fuse to form larger intracellular crystals (re-crystallization) that enhances the mechanical damage to the cell membranes and intracellular organelles. (C) Post-thawing, mechanically damaged cells die by necrosis and release their contents into the surrounding milieu. Cells that have undergone exosmosis swell and burst due to osmotic shock. Cells in the utmost periphery of the ablation zone exposed to sub-lethal temperatures undergo apoptosis, releasing apoptotic bodies. Antigens released from necrotic cells upon uptake by antigen presenting cells like DCs, induce co-stimulatory signals that would result in the generation of anti-tumoral T-cell responses. In contrast, antigen uptake by DCs in the form of apoptotic bodies imprints immune tolerance or anergy on T-cells due to the non-induction of co-stimulatory signals on DCs. DC, Dendritic cell.