Abstract
Objective:
To investigate the role of miR-26a-5p in cell proliferation and doxorubicin sensitivity in hepatocellular carcinoma.
Methods:
We evaluated miR-26a-5p expression in hepatocellular carcinoma tissues and cell lines by reverse transcription polymerase chain reaction. Cell Counting Kit-8 was used to examine cell proliferation. Relationship between miR-26a-5p and aurora kinase A was evaluated by luciferase report system. Western blot was used to detect expression of aurora kinase A.
Results:
In this study, we observed miR-26a-5p was downregulated in hepatocellular carcinoma tissues and cell lines. Gain-of-function experiments showed that proliferation rate of hepatocellular carcinoma cells decreased under condition of miR-26a-5p mimics. We found miR-26a-5p mimics could enhance doxorubicin sensitivity of hepatocellular carcinoma cells. Further study showed that aurora kinase A was target gene of miR-26a-5p. Suppression of aurora kinase A could lead to lower cell proliferation and higher doxorubicin sensitivity of hepatocellular carcinoma cells.
Conclusion:
Our study found that miR-26a-5p could inhibit cell proliferation and enhance doxorubicin sensitivity in hepatocellular carcinoma cells by targeting aurora kinase A.
Keywords: MiR-26a-5p, hepatocellular carcinoma (HCC), aurora kinase A (AURKA), doxorubicin sensitivity
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and has persistently increasing the rates of both incidence and mortality.1 Only patients with early HCC are eligible for surgical resection, liver transplantation, or local ablation; however, those diagnosed with intermediate and advanced HCC may only obtain chemotherapy to protect tumor progression.2-4 Transarterial chemoembolization is currently considered the only sufficient treatment for patients with advanced HCC, and doxorubicin is an anthracycline-based agent that is widely used as an anti-HCC drug systematically or locally.5,6 However, chemoresistance is an insurmountable gap during long-term doxorubicin treatment, which leads to recurrence and poor prognosis.1,5 Thus, more studies about the mechanism of drug resistance in HCC are necessary.
MicroRNAs (miRNAs) are evolutionary conserved small RNAs that posttranscriptionally regulate gene expression via targeting the 3′-untranslated region (3′-UTR) of messenger RNAs (mRNAs).7 It has been proved that many miRNAs are correlated with drug resistance of human cancers8; thus, development of miRNA-based therapeutic strategy using the functional miRNA regulators may be more effective for cancer treatment. Recent study shows that miR-26a-5p was downregulated in HCC serum and was one of the biomarkers used in diagnosing hepatitis B virus–related HCC.9 Based on these evidence, we supposed that miR-26a-5p may not only be associated with progression of HCC but also play an important role in drug resistance. Our study aims to investigate function of miR-26a-5p in regulating cell proliferation and doxorubicin resistance in HCC cells.
Materials and Methods
Patients and Tissue Samples
All cancer tissue samples and neighbor tissue samples were obtained from 35 patients with HCC who underwent surgery at the People’s Hospital of Zhengzhou University. All patients were diagnosed with HCC by imaging and serological examination and accepted operation without radiation and chemotherapy. The Medical Ethics Committee of People’s Hospital of Zhengzhou University approved the protocol, and written informed consent was obtained from all participants. Fresh cancer tissues and adjacent matched normal tissues were collected and stored at −80 C.
Cell Culture
The HCC cell lines (Huh7, HCC-LM3, Hep-G2, Hep-3B and SMMC-7721) and normal immortalized hepatocytes (QSG-7701) were purchased from the Chinese Academy of Science Cell Bank (Shanghai, China). All these cells were stored in MEM medium and supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2.
Transfection of Huh7 and SMMC-7721 Cell Lines
MiR-26a-5p mimics, inhibitors, and negative controls were synthesized by Qiagen (Hilden, Germany). Human-specific aurora kinase A (AURKA) interference RNA (siRNA) was designed, constructed, and purified by Jima Biotech Co (Shanghai, China). The siRNA targeting AURKA was the following sequence: 5′-UUCUUAGACUGUAUGGUUA-3′. Human AURKA complementary DNA (cDNA) clone was purchased from Origene (CAT#: RC212018). The transfection was conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, California), according to the manufacturer’s instruction.
Real-Time Polymerase Chain Reaction Analysis
Total RNA was extracted from Huh7 and SMMC-7721 cell lines using TRIzol reagent following the manufacturer’s protocol (Invitrogen). First-strand cDNA was generated using a reverse transcription system kit (Promega Corporation, Madison, Wisconsin). The primers of miR-26a-5p was purchased from TAKARA (Dalian, China). Expression of miR-26a-5p was measured by quantitative polymerase chain reaction with ABI7500 Fast System (Applied Biosystems, Carlsbad, California) and SYBR green dye (TAKARA Biotechnology). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal controls, and the 2−△△Ct method was used for relative quantification. All reactions were performed in triplicate.
Luciferase Reporter Assay
Huh7 and SMMC-7721 cells were seeded in 24-well plates and then the cells were cotransfected with 5 µL miR-26a-5p, anti-miR-26a-5p, or control with concentration of 10 nM and 100 ng either wild-type or mutant-type 3′-UTR of AURKA firefly luciferase reporter plasmid. After incubation for 48 hours, firefly and Renilla luciferase activities were measured by a dual-luciferase reporter assay (Promega E2920).
Western Blot Analysis
Total proteins were extracted from harvested cells by protein extract kit (Beyontime, Jiangsu, China). All the proteins were degenerated by boiling, separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membranes. Then membranes were blocked with 5% milk. Next, the membranes were incubated into first antibodies overnight at 4°C. Then washing 3 times with Tris-buffered saline Tween 20, the membranes were incubated by second antibody (goat anti-rabbit) for 2 hours under 37°C incubator. Finally, all the membranes were visualized by a chemiluminescence kit (Beyontime) on a Bio-Rad imaging system. Primary antibodies used in this study are as follows: AURKA (Proteintech, 10297-1-AP, dilution 1/1000) and the internal control was GAPDH (Proteintech, 60004-1-Ig, dilution 1/3000).
Cell Viability
To detect the relative cell viability, Huh7 and SMMC-7721 cell lines were seeded into 96-well microplates at a density of 5000 cells per well with or without different concentrations of doxorubicin. Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan) was then performed according to the manufacturer’s instructions at 24, 48, and 72 hours. Briefly, 10 μL of CCK-8 working solution per 100 μL of medium was added into the microplates and the cells were incubated for 2 hours. The OD450 value was determined using an MRX II microplate reader (Dynex, Chantilly, Virginia).
Statistical Analysis
Data are presented as means ± standard deviation (SD). For comparisons, the Student t test, paired-samples t test, and analysis of variance were performed as appropriate. All analyses were performed with SPSS 18.0 software (SPSS Inc, Chicago, Illinois). Differences were considered significant at P < .05.
Results
MiR-26a-5p Was Downregulated in HCC Tissues and Cell Lines
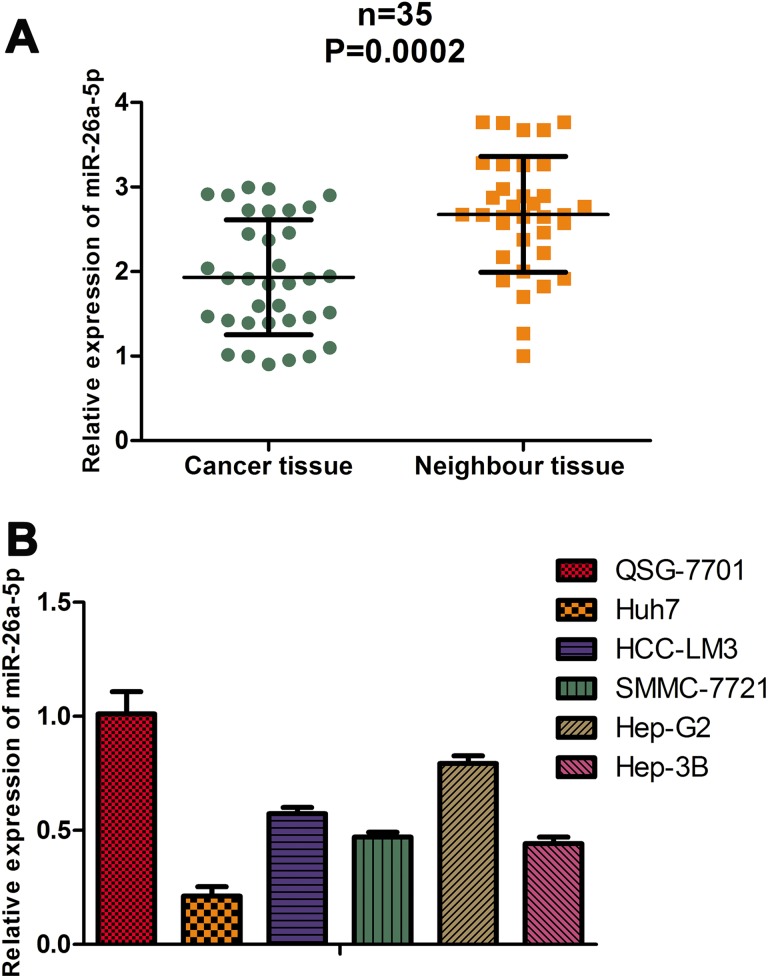
We first detected expression of miR-26a-5p in 35 paired HCC tissues and neighbor tissues, and the results showed that miR-26a-5p was significantly decreased in HCC tissues (Figure 1A). Then we examined expression of miR-26a-5p in HCC cell lines (Huh7, HCC-LM3, SMMC-7721, Hep-G2, and Hep-3B) and normal immortalized hepatocyte (QSG-7701) and found miR-26a-5p was higher expression in QSG-7701 than all the HCC cell lines (Figure 1B).
Figure 1.
miR-26a-5p is downregulated in hepatocellular carcinoma (HCC) tissues and cell lines. A, Expression level of miR-26a-5p is detected in 35 paired HCC tissues and neighbor tissues, and the result is analyzed by the Student t test. P < .05 means statistical significance. B, Expression level of miR-26a-5p is detected in HCC cell lines and normal immortalized hepatocyte.
MiR-26a-5p Inhibited Cell Proliferation and Enhanced Doxorubicin Sensitivity in HCC Cells
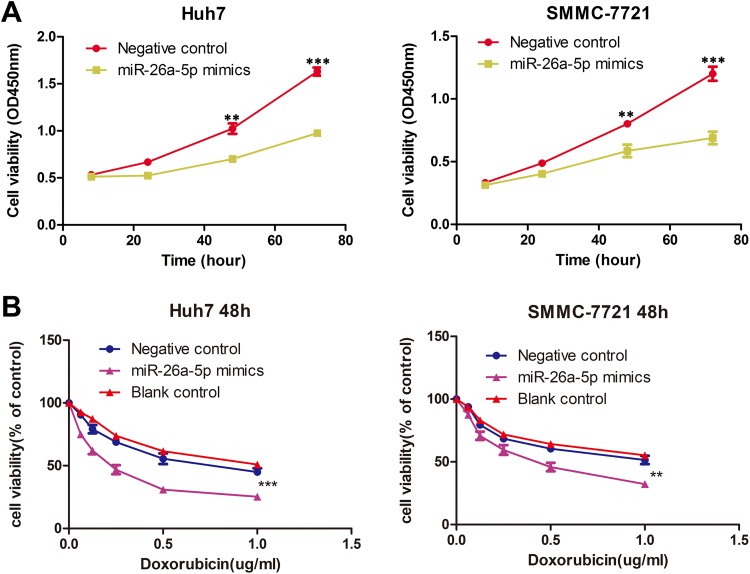
To investigate function of miR-26a-5p in HCC cells, we first detected cell proliferation under condition of miR-26a-5p mimics and negative control, and the results showed that miR-26a-5p could decrease the proliferation rate of HCC cells (Figure 2A). We explored function of miR-26a-5p on doxorubicin sensitivity in HCC cells, and the results displayed that miR-26a-5p could enhance doxorubicin sensitivity on HCC cell lines (Figure 2B).
Figure 2.
miR-26a-5p inhibits proliferation and enhances doxorubicin sensitivity in hepatocellular carcinoma (HCC) cells. A, Cell viability of Huh7 and SMMC-7721 at 48 and 72 hours was compared between miR-26a-5p mimics and negative control; the result was analyzed by the Student t test, ***P < .0005. B, Cell viability of Huh7 and SMMC-7721 was detected under different concentrations (0, 0.0625, 0.125, 0.25, 0.5, and 1.0 µg/mL) of doxorubicin. And the results were analyzed by analysis of variance (ANOVA),***P < .0005,** P < .005.
Aurora Kinase A Targeting Was Regulated by miR-26a-5p in HCC Cells
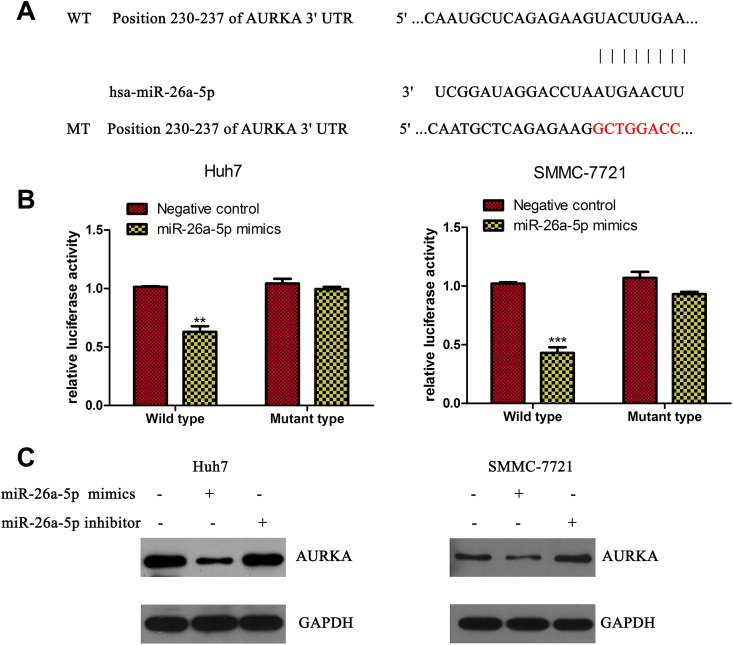
To investigate the downstream gene of miR-26a-5p in HCC cells, we used TargetScan database to predict the target gene. As miR-26a-5p was demonstrated to have an important role in cell proliferation and doxorubicin sensitivity, and AURKA was reported to be associated with cell proliferation and drug resistance in HCC cells,10 we supposed that miR-26a-5p could regulate cell proliferation and doxorubicin sensitivity via targeting AURKA in HCC cells. Then we constructed wild-type and mutant-type AURKA 3’UTR according to sequence of miR-26a-5p and designed luciferase reporter system to validate our supposition (Figure 3A). The results of luciferase report experiment showed that miR-26a-5p could be targeting combination with 3′UTR of AURKA mRNA (Figure 3B). To validate such results, we detected protein level of AURKA with treatment of miR-26a-5p mimics or inhibitor, and the results showed that AURKA was decreased under condition of miR-26a-5p while almost similar in negative control group and miR-26a-5p inhibitor group (Figure 3C).
Figure 3.
Aurora kinase A (AURKA) is a target gene regulated by miR-26a-5p. A, TargetScanHuman 7.1 showed AURKA 3′ untranslated region (3′UTR) had complementary pairing with miR-26a-5p, and we constructed the wild-type and mutant-type AURKA 3′UTR. B, The results of luciferase activity experiment in Huh7 and SMMC-7721,** P < .005,***P < .0005. C, Protein level of AURKA when treated with miR-26a-5p mimics or miR-26a-5p inhibitor; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is used as an internal control.
MiR-26a-5p Regulated Cell Proliferation and Doxorubicin Sensitivity by Targeting AURKA in HCC Cells
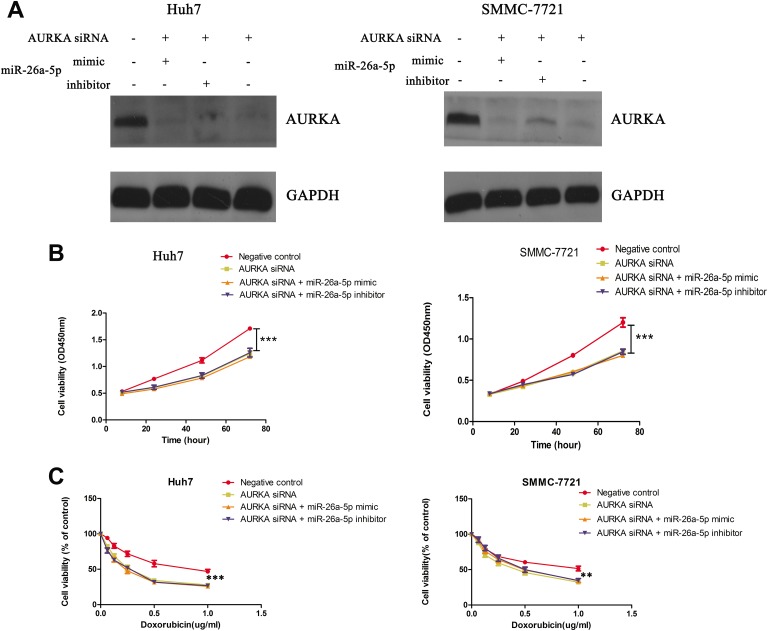
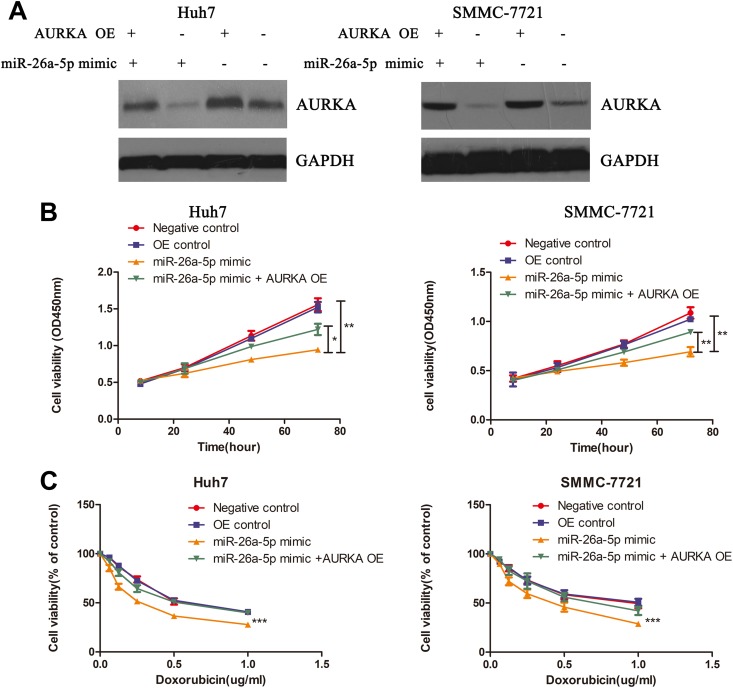
To demonstrate whether miR-26a-5p regulated cell proliferation and doxorubicin sensitivity by targeting AURKA, we used AURKA siRNA to suppress expression of AURKA in HCC cell lines and observed that expression of AURKA among AURKA siRNA alone, miR-26a-5p mimics + AURKA siRNA, and miR-26a-5p inhibitor + AURKA siRNA groups was almost similar (Figure 4A). Based on this, we observed that the cell proliferation had almost no change among AURKA siRNA, miR-26a-5p mimics + AURKA siRNA, and miR-26a-5p inhibitor + AURKA siRNA groups, and the proliferation rate of negative control was higher than the abovementioned 3 groups. (Figure 4B). In addition, we found suppression of AURKA could significantly increase the doxorubicin sensitivity in HCC cells, whether or not with miR-26a-5p mimics or miR-26a-5p inhibitors (Figure 4C). To further prove these results, we constructed AURKA overexpression (OE) plasmid, and the transfection effect was effective and could reverse the efficiency of miR-26a-5p mimics (Figure 5A). Then we detected cell viability in HCC cells with miR-26a-5p mimics, miR-26a-5p mimics +AURKA OE, OE control, and negative control by CCK-8 assay. The results showed that cell viability of HCC cells with miR-26a-5p mimics alone was lowest among these groups, and AURKA OE could reverse the effect of miR-26a-5p mimics (Figure 5B). We also found doxorubicin sensitivity of HCC cells with AURKA OE + miR-26a-5p mimics was lower than those with miR-26a-5p mimics alone (Figure 5C). Based on these, we demonstrated miR-26a-5p could inhibit cell proliferation and enhance doxorubicin sensitivity by targeting AURKA in HCC cells.
Figure 4.
miR-26a-5p regulates proliferation and doxorubicin sensitivity via targeting aurora kinase A (AURKA). A, Protein level of AURKA in hepatocellular carcinoma (HCC) cells is detected among groups of negative control, AURKA siRNA, miR-26a-5p mimics + AURKA siRNA, and miR-26a-5p inhibitor + AURKA siRNA. B and C, Cell viability of HCC cells is detected among groups of negative control, AURKA siRNA, miR-26a-5p mimics + AURKA siRNA, and miR-26a-5p inhibitor + AURKA siRNA, under different times (24, 48, and 72 hours) or different concentrations of doxorubicin (0, 0.0625, 0.125, 0.25, 0.5, and 1.0 µg/mL). Analysis of variance (ANOVA) was used to analyze statistical difference. ***P < .0005, **P < .005.
Figure 5.
Overexpression of aurora kinase A (AURKA) can reverse the effect of miR-26a-5p in hepatocellular carcinoma (HCC) cells. A, Protein level of AURKA in HCC cells is detected among groups of negative control, miR-26a-5p mimics, and miR-26a-5p mimics + AURKA overexpression (OE). B and C, Cell viability of HCC cells is detected among groups of negative control, OE control, miR-26a-5p mimics, and miR-26a-5p mimics + AURKA OE under different times (24, 48, and 72 hours) or different concentration of doxorubicin (0, 0.0625, 0.125, 0.25, 0.5, and 1.0 µg/mL), Analysis of variance (ANOVA) was used to analyze statistical difference. *P < .05, **P < .005,***P < .0005.
Discussion
MicroRNAs have been demonstrated to play an important role in tumor proliferation, migration, and drug resistance via targeting different kinds of mRNA.11 And recent studies have reported that miR-26a-5p played a critical role in regulating cell proliferation, migration, and aggressiveness in various tumors, such as gallbladder cancer,12 bladder cancer,13 acute myeloid leukemia,14 colorectal carcinoma,15 prostate cancer,16 neuroblastoma,17 non-small cell lung cancer18, hepatoblastoma19 and HCC.9 And serum miR-26a-5p level was proved to be potential biomarker for HCC,9 prostate cancer,20 and non-small cell lung cancer.18 In addition, miR-26a-5p could suppress metastasis of HCC by regulating epithelial-mesenchymal transition (EMT), which was demonstrated to be associated with chemoresistance in HCC.21,22 Based on these evidences, we hypothesized that miR-26a-5p may play an important role in proliferation and drug resistance in HCC.
In this study, we observed that miR-26a-5p was downregulated in HCC tissues and cell lines. Gain-of-function experiment showed that miR-26a-5p mimics could decrease the proliferation rate and enhance doxorubicin sensitivity in HCC cells. To further investigate the downstream gene of miR-26a-5p in HCC cells, we used TargetScanHuman 7.1 software to predict the candidate gene. Then, we found AURKA targeting was regulated by miR-26a-5p. Recent studies have demonstrated that AURKA was associated with cell cycle progression, epithelial–mesenchymal transition, and cancer stem cell properties in HCC.10,23-25 Based on these, we hypothesized that miR-26a-5p may regulate proliferation and doxorubicin sensitivity via targeting AURKA.
Then we used luciferase system to investigate whether miR-26a-5p could regulate expression of AURKA in HCC cells. We observed that miR-26a-5p mimics could significantly decrease the luciferase activity in AURKA 3′UTR wild-type group. Furthermore, we observed that the expression of AURKA could be decreased under condition of miR-26a-5p mimics while no change with miR-26a-5p inhibitor. To further demonstrate our discovery, we used siRNA to suppress expression of AURKA in HCC cells. Then we observed that the efficiency of AURKA siRNA on AURKA expression, cell proliferation, and doxorubicin sensitivity was almost the same as the “miR-26a-5p mimic +AURKA siRNA” group and “miR-26a-5p inhibitor +AURKA siRNA” group. In addition, we transfected AURKA ORF clone plasmid into HCC cells and found OE of AURKA could reverse the AURKA suppression, low cell proliferation rate, and high doxorubicin sensitivity induced by miR-26a-5p. Thus, we proved that miR-26a-5p could regulate proliferation and doxorubicin sensitivity of HCC cells via targeting AURKA.
In conclusion, our study proved that miR-26a-5p was associated with proliferation and doxorubicin sensitivity of HCC cells. And AURKA was the downstream target gene of miR-26a-5p. However, our clinical sample is too small to prove whether miR-26a-5p is a potential new therapeutic target for patients with advanced HCC. Further studies are needed to verify our findings.
Acknowledgments
The authors thank all the patients, their families, the investigators, co-medical staffs, and all others who participated in the present study.
Abbreviations
- AURKA
aurora kinase A
- CCK-8
Cell Counting Kit-8
- cDNA
complementary DNA
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HCC
hepatocellular carcinoma
- miRNAs
microRNAs
- mRNA
messenger RNA
- OE
overexpression
- siRNA
human-specific AURKA interference RNA
- 3′UTR
3′-untranslated region.
Authors’ Note: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Our study was approved by the Medical Ethics Committee of People’s Hospital of Zhengzhou University (approval no.2018112). All patients provided written informed consent prior to enrollment in the study. Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was funded by the basic and frontier of Henan Science and Technology Department (122300410065) and Henan Provincial Health Department Guiding Plan (201204092).
ORCID iD: De Yu Li, MD  https://orcid.org/0000-0002-6365-7611
https://orcid.org/0000-0002-6365-7611
Reference
- 1. Finn RS. Current and future treatment strategies for patients with advanced hepatocellular carcinoma: role of mTOR inhibition. Liver cancer. 2012;1(3-4):247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou Y, Liang C, Xue F, et al. Salinomycin decreases doxorubicin resistance in hepatocellular carcinoma cells by inhibiting the β-catenin/TCF complex association via FOXO3a activation. Oncotarget. 2015;6(12):10350–10365. 03/14 12/21/received 02/13/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abou-Alfa GK. Sorafenib use in hepatocellular carcinoma: more questions than answers. Hepatology. 2014;60(1):15–18. [DOI] [PubMed] [Google Scholar]
- 4. Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer lett. 2014;347(2):159–166. [DOI] [PubMed] [Google Scholar]
- 5. Forner A, Gilabert M, Bruix J, Raoul J-L. Treatment of intermediate-stage hepatocellular carcinoma. Nat rev Clin oncol. 2014;11(9):525–535. [DOI] [PubMed] [Google Scholar]
- 6. Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. JPharm Pharmacol. 2013;65(2):157–170. [DOI] [PubMed] [Google Scholar]
- 7. Hong L, Han Y, Li S, et al. The malignant phenotype-associated microRNA in gastroenteric, hepatobiliary and pancreatic carcinomas. Expert opin biol th. 2010;10(12):1693–1701. [DOI] [PubMed] [Google Scholar]
- 8. Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer. 2010;126(1):2–10. [DOI] [PubMed] [Google Scholar]
- 9. Tan Y, Ge G, Pan T, et al. A serum microRNA panel as potential biomarkers for hepatocellular carcinoma related with hepatitis B virus. PloS One. 2014;9(9):e107986 09/19 06/12/received 08/16/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen C, Song G, Xiang J, Zhang H, Zhao S, Zhan Y. AURKA promotes cancer metastasis by regulating epithelial-mesenchymal transition and cancer stem cell properties in hepatocellular carcinoma. Biochem bioph res co. 2017;486(2):514–520. [DOI] [PubMed] [Google Scholar]
- 11. Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat rev Genet. 2009;10(10):704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang SH, Yang Y, Wu XC, et al. Long non-coding RNA MINCR promotes gallbladder cancer progression through stimulating EZH2 expression. Cancer Lett. 2016;380(1):122–133. [DOI] [PubMed] [Google Scholar]
- 13. Miyamoto K, Seki N, Matsushita R, et al. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Brit J Cancer. 2016;115(3):354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang W, Min J, Sui X, et al. MicroRNA-26a-5p and microRNA-23b-3p up-regulate peroxiredoxin III in acute myeloid leukemia. Leukemia lymphoma. 2015;56(2):460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghanbari R, Mosakhani N, Asadi J, et al. Downregulation of plasma MiR-142-3p and MiR-26a-5p in patients with colorectal carcinoma. Iran J Cancer Prev. 2015;8(3):e2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rizzo M, Berti G, Russo F, et al. Discovering the miR-26a-5p targetome in prostate cancer cells. J Cancer. 2017;8(14):2729–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beckers A, Van Peer G, Carter DR, et al. MYCN-driven regulatory mechanisms controlling LIN28B in neuroblastoma. Cancer lett. 2015;366(1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leidinger P, Brefort T, Backes C, et al. High-throughput qRT-PCR validation of blood microRNAs in non-small cell lung cancer. Oncotarget. 2016;7(4):4611–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Zhao Y, Wu J, Liangpunsakul S, Niu J, Wang L. MicroRNA-26-5p functions as a new inhibitor of hepatoblastoma by repressing lin-28 homolog B and aurora kinase a expression. Hepatol commun. 2018;2(7):861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cochetti G, Poli G, Guelfi G, Boni A, Egidi MG, Mearini E. Different levels of serum microRNAs in prostate cancer and benign prostatic hyperplasia: evaluation of potential diagnostic and prognostic role. OncoTargets ther. 2016;9:7545–7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang LLK, Guo T., miR-26a-5p suppresses tumor metastasis by regulating EMT and is associated with prognosis in HCC. Clin Transl Oncol. 2017;19(6):695–703. [DOI] [PubMed] [Google Scholar]
- 22. Chen XLS, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J hepatol. 2011;55(4):838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H, Li R, Li L, Ge Z, Zhou R, Li R. LRD-22, a novel dual dithiocarbamatic acid ester, inhibits Aurora-A kinase and induces apoptosis and cell cycle arrest in HepG2 cells. Biochem bioph res co. 2015;458(1):201–207. [DOI] [PubMed] [Google Scholar]
- 24. Lu L, Han H, Tian Y, et al. Aurora kinase A mediates c-Myc’s oncogenic effects in hepatocellular carcinoma. Mol carcinogen. 2015;54(11):1467–1479. [DOI] [PubMed] [Google Scholar]
- 25. Dauch D, Rudalska R, Cossa G, et al. A MYC–aurora kinase a protein complex represents an actionable drug target in p53-altered liver cancer. Nat med. 2016;22(7):744. [DOI] [PubMed] [Google Scholar]