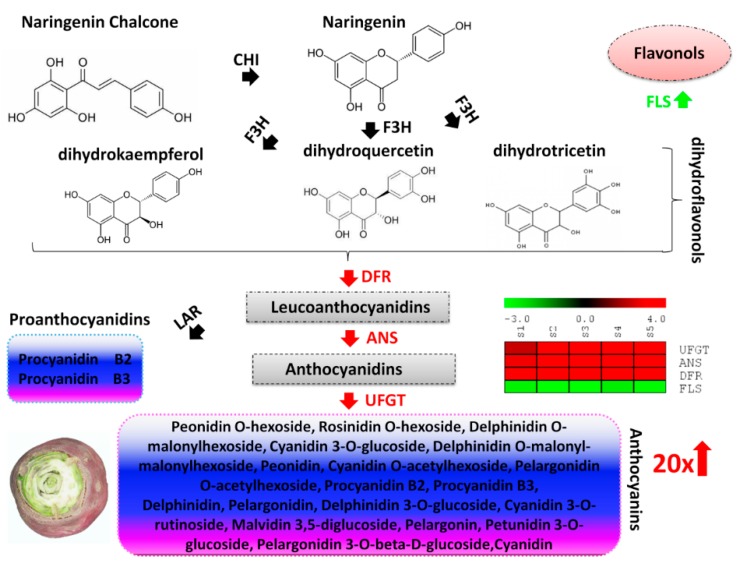
Figure 5.
Proposed model of the molecular mechanism leading to the high anthocyanin content in the the purple turnip (PT). Naringenin chalcone is isomerized by chalcone isomerase (CHI) to naringenin. Flavanone 3-hydroxylase (F3H) converts naringenin into dihydroflavonols (dihydrokaempferol, dihydroquercetin or dihydrotricetin). Then, the three dihydroflavonols are converted into colorless leucoanthocyanidins by dihydroflavonol 4-reductase (DFR) and subsequently to colored anthocyanidins by anthocyanidin synthase (ANS). Anthocyanidins are glycolsylated to facilitate their accumulation in cells by the enzyme flavonoid 3-O-glucosyltransferase (UFGT). Proanthocyanidins are generated by the action of leucoanthocyanidin reductase (LAR) from leucoanthocyanidins. DFR, ANS and UFGT were found significantly up-regulated in PT leading to a high content of 17 anthocyanins compounds (more than 20 times compared to the green turnip). In contrast, FLS was found significantly down-regulated and may lead to a weak accumulation of flavonols. PT tends to prioritize anthocyanins accumulation by diverting dihydroflavonols to the anthocyanins biosynthesis pathway.