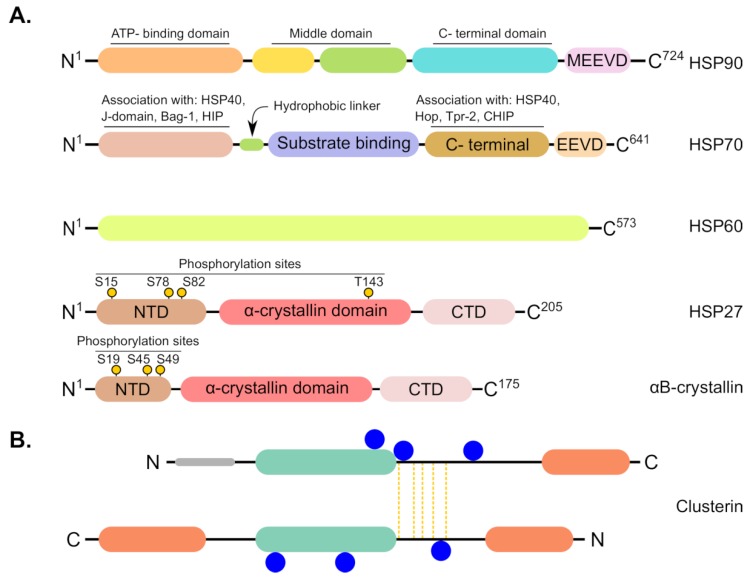
Figure 4.
Schematic representation showing structural features of common chaperones involved in ovarian cancer. (A) From top to bottom; HSP90, HSP70 and HSP60 are ATP-dependent chaperones that harbor ATP binding sites within their structures whereas sHSPs such as HSP27 and αB-crystallin do not possess ATP binding sites. All HSPs have N-terminal and C-terminal domains (NTD and CTD) besides middle domain. sHSPs contain phosphorylation sites at specific serine (S) or threonine (T) residues, depicted as black sticks with yellow circles at their ends, and they are characterized by conserved α-crystallin domain that is flanked by variable N- and C-terminal ends. (B) The main structural topology of clusterin (CLU). Synthesis of CLU includes removal of the short 22-residue signal peptide (grey) as it enters to the ER lumen. Subsequent posttranslational proteolysis occurs in the Golgi where the protein is cleaved into α-(upper) and β-(lower) chains. The α- and β-chains are covalently connected by five disulfide bridges extending from ‘core region’ of both chains (yellow vertical lines). In addition, the α-chain is predicted to contain one amphipathic α-helix while the β-chain contains two α-helices. Moreover, both chains have a coiled coil structure (light green) and the mature protein is known to have six N-linked glycosylation sites (blue circles).