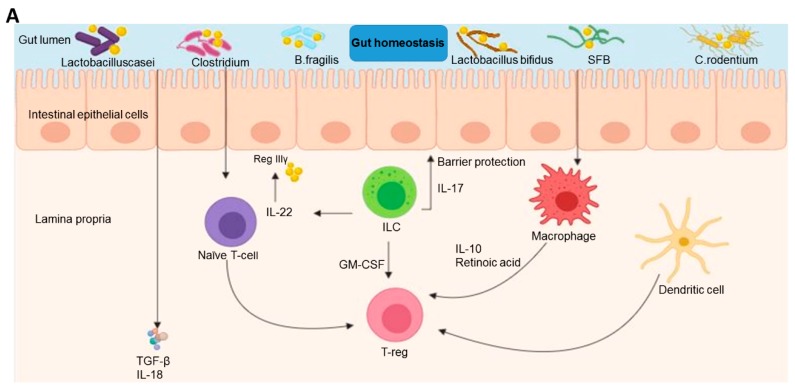
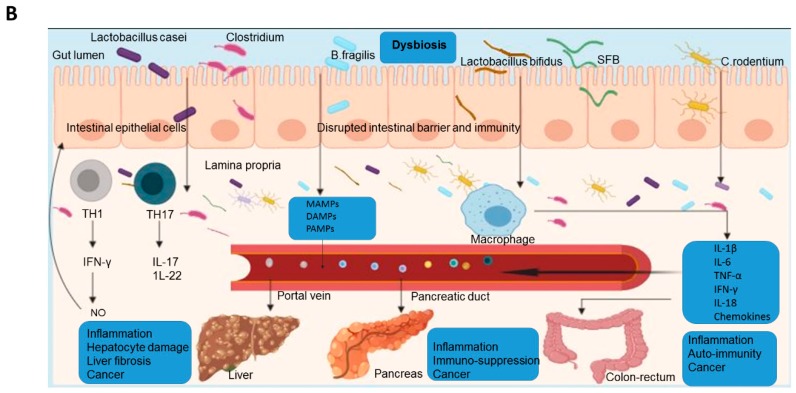
Figure 1.
Intestinal homeostasis and dysbiosis of gut microbiomes in gastrointestinal cancer development. (A) Gut homeostasis. The gut homeostasis is maintained by an intricate network of factors such as regenerating islet-derived III-gamma (RegIIIγ), interleukin-22 (IL-22) and interleukin-17 (IL-17) secreted by innate lymphoid cells (ILC). These factors regulate the diverse gut microbiome, aid tissue repair, and barrier protection. Inhibition of immune activation by regulatory T cells (Treg) is aided by interleukin-10 (IL-10), retinoic acid and granulocyte macrophage-colony stimulating factor (GM-CSF) secreted by macrophage and innate lymphoid cells (ILC) respectively. In addition, secreted TGF-β and IL-18 preserve the intestinal barrier integrity and also promotes early development of regulatory T cells (Tregs). (B) Dysbiosis. On the other hand, an altered gut barrier due to a dysregulated microbiome disrupts the intestinal barrier resulting to leakage of microorganism associated molecular patterns (MAMPs), pathogens associated molecular patterns (PAMPs) and death associated molecular patterns (DAMPs) into the lamina propria. In addition, T helper-1 and T helper-17 produce interferon-gamma (IFN-γ), interleukin-17 (IL-17), interleukin -22 (IL-22) and excess nitric oxide, which induce loss of barrier integrity. Macrophages secretes inflammatory factors such as tumor necrosis factor (TNF-α), interferon- gamma (IFN-γ), interleukin-1 beta (IL-1β), interleukin -6 (IL-6), interleukin -16 (IL-16), interleukin-17 (IL-17), interleukin-18 (IL-18), and chemokines. These factors translocate via the portal vein and pancreatic duct to the liver and pancreas, respectively, and do so directly unto the colon–rectum, thereby initiating inflammation and cancer.