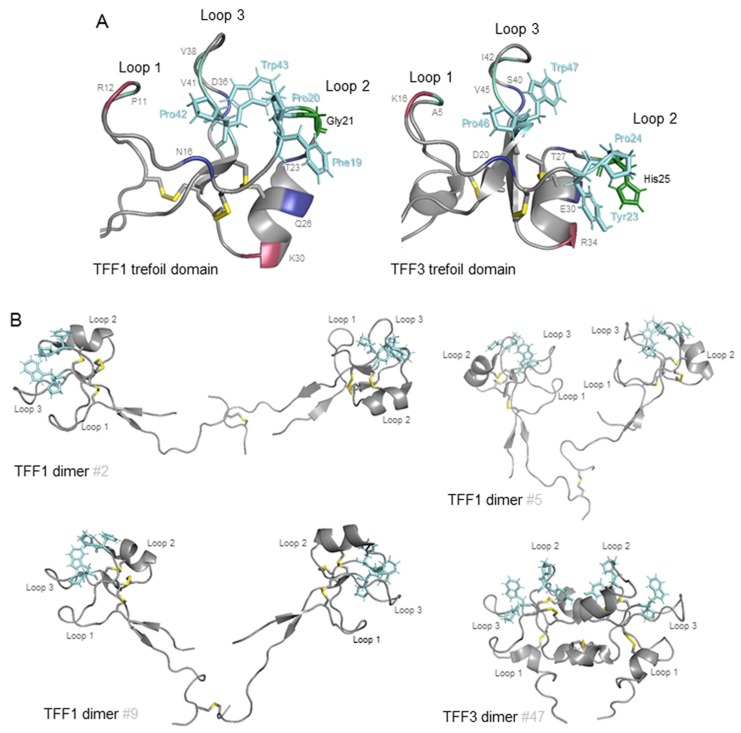
Figure 3.
Hydrophobic surfaces of TFF1 and TFF3. Representations of the backbones of the TFF1 and TFF3 trefoil domains [57,60] (A) and TFF1 and TFF3 dimers [58,59] (B) are shown. The intramolecular and intermolecular disulphide bonds are shown and coloured yellow. The three fully conserved solvent accessible residues, Pro 20, Pro42 and Trp43 in TFF1, and one semi-conserved solvent accessible residue, Phe19 in TFF1, in loops 2 and 3 that form a continuous hydrophobic patch postulated to interact with a saccharide or aromatic amino acid sidechain between loops 2 and 3 are shown in stick representation and are coloured cyan (A,B). The backbones of the surface residues with conserved features that surround this patch are indicated in single letter code (A). Of these, the hydrophobic residues, Pro11 (P11), Val38 (V38) and Val41 (V41) in TFF1, are coloured pale green, the hydrogen bond donor, Arg12 (R12) is coloured raspberry red and the hydrogen bond acceptors, Asn16 (N16), Thr23 (T23), Gln26 (Q26) and Asp36 (D36) in TFF1, are coloured deep blue. Two other well-conserved residues on the edge of the hydrophobic patch are indicated. A positively charged residue, Lys30 (K30) in TFF1, is coloured raspberry red. The small Gly21 in TFF1 that would facilitate access to the hydrophobic cleft and the corresponding His25 residue of TFF3 proposed to impede access are shown in stick representation and are coloured forest green (A). Three of the solution NMR structures of the TFF1 dimer, #2, #5 and #9 and one of the TFF3 dimers (#47), are shown with the first monomer unit on the left-hand side.