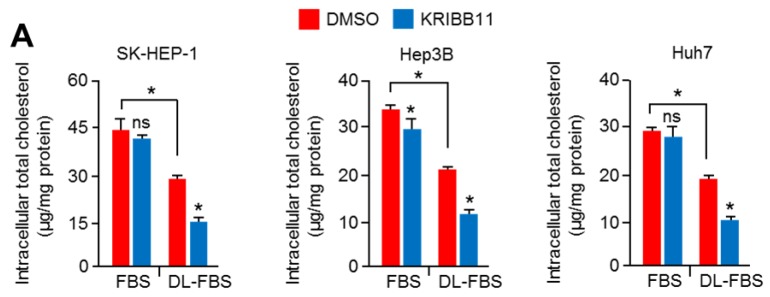
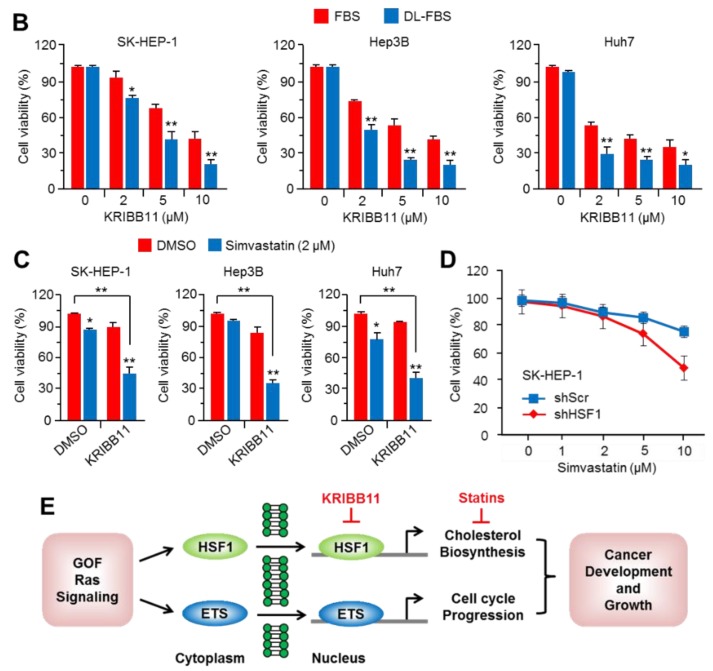
Figure 6.
HSF1 inhibition sensitized the suppression of HCC cell growth in cholesterol-depleted conditions. (A) SK-HEP-1, Hep3B, and Huh7 were incubated for 24 h in normal FBS or DL-FBS in the absence or presence of KRIBB11 (5 μM), and then cells were used to measure intracellular cholesterol levels. The values represent the mean ± SD of three independent experiments performed in duplicate. (B) SK-HEP-1, Hep3B, and Huh7 were incubated for 48 h in normal FBS or DL-FBS in the absence or presence of various concentrations of KRIBB11 (2, 5, and 10 μM), as indicated. Cell viability was measured by crystal violet staining. The values represent the mean ± SD from three independent experiments performed in duplicate. (C) SK-HEP-1, Hep3B, and Huh7 were incubated for 48 h in Dimethyl sulfoxide (DMSO) (as a control) or simvastatin (2 μM) in the absence or presence of KRIBB11 (5 μM). The values represent the mean ± SD from three independent experiments performed in duplicate. (D) HSF1 knocked-down SK-HEP-1 cells were incubated with various concentrations of simvastatin (1, 2, 5, and 10 μM) for 48 h, as indicated. The values represent the mean ± SD from three independent experiments performed in duplicate. (E) Proposed molecular mechanism by which HSF1 and erythroblast transformation-specific (ETS) transcription factor activated by gain-of-function (GOF) RAS-MAPK signaling promotes cholesterol biosynthesis and cell cycle progression, and this regulatory axis may be involved in cancer development and progression. * p < 0.05; ** p < 0.01; ns, not significant.