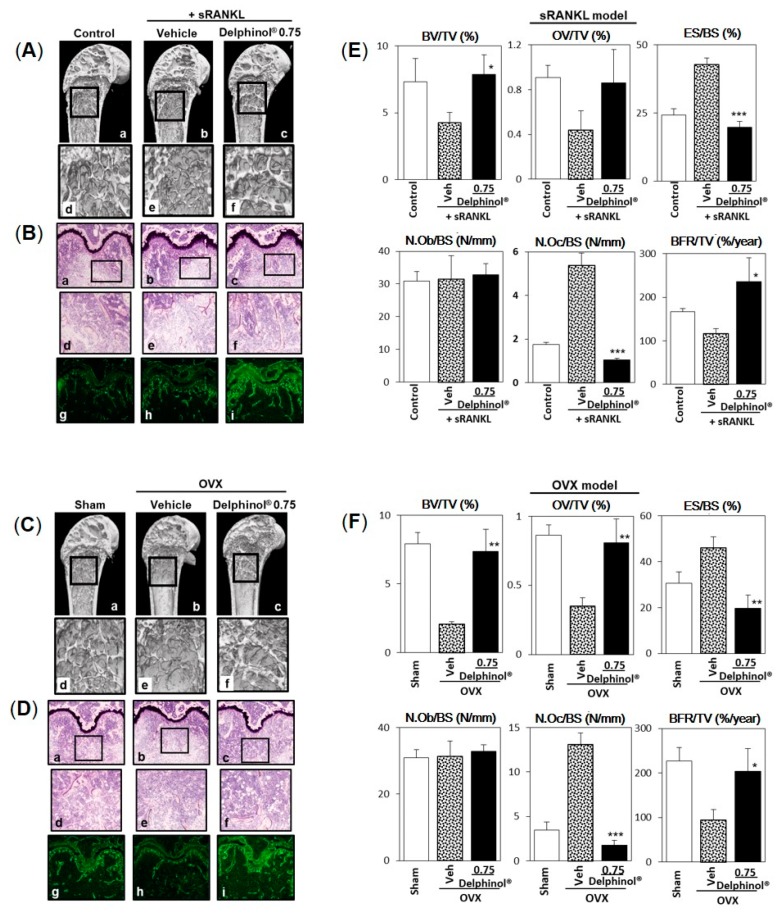
Figure 7.
Histomorphometrical analyses of effect of Delphinol® on bone loss in sRANKL-induced and OVX-induced osteopenic mouse models. (A) Representative three-dimensional micro-CT images of the sagittal midsection of distal end of femurs in intact mice (Figure 7(Aa,Ad), control), sRANKL-induced osteopenic mice (Figure 7(Ab,Ae), vehicle), and Delphinol® 0.75 (mg/mouse/day)-administered sRANKL-induced osteopenic mice (Figure 7(Ac,7Af)). (C) Representative micro-CT images of the sagittal midsection of distal femurs in sham-operated mouse (Sham), OVX-induced osteopenic mouse (vehicle), and a Delphinol® 0.75 (mg/mouse/day)-treated OVX-induced osteopenic mouse. (B,D) Representative photographs of distal femur sections stained by Villanueva staining in sRANKL- and OVX-induced osteopenic mice, respectively. Figures in the middle panel correspond to the squares in the upper panel. Figures in the lowest panel are fluorescent (calcein) images of the same field of Villanueva staining. (E,F) Histomorphometric parameters were determined by morphometric analyses of fluorescent (tetracycline and calcein)-labeled femurs according to the methods of the ASBMR Histomorphometry Nomenclature Committee [44]. Values are expressed as mean ± SD (n = 5).; Significance was tested between vehicle and 0.75 mg/mouse/day Delphinol®. *p < 0.05, **p < 0.01, ***p < 0.001.