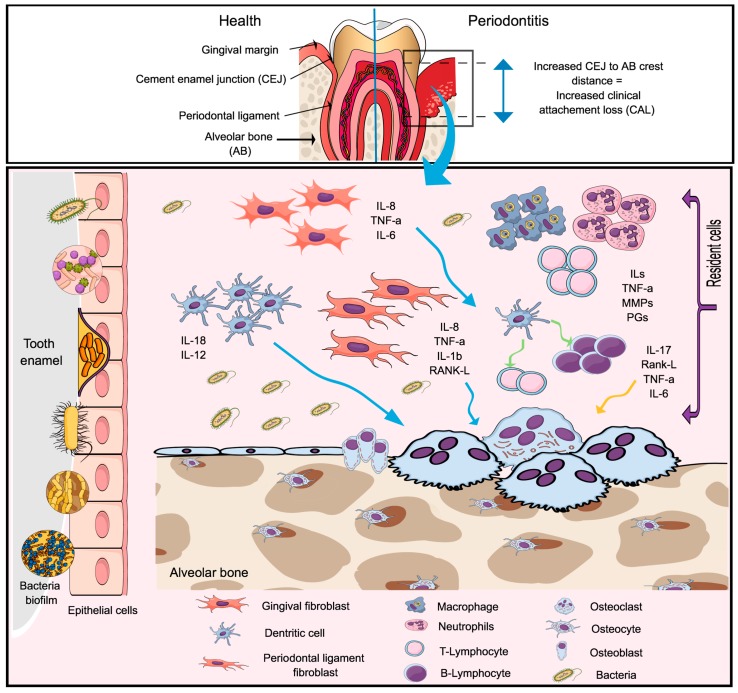
Figure 2.
The pathogenesis of the periodontal disease. A dysbiotic microbiome localized in the enamel surface of the tooth, below the gingival margin, initiate the innate immunity by stimulating resident cells (epithelial cells, periodontal ligament fibroblast, and gingival fibroblast and dendritic cells) to produce mediators of inflammation in response to bacterial lipopolysaccharide (LPS) (via the toll-like receptor). Resident cells located in the connective tissue and alveolar bone produce proinflammatory cytokines and chemokines, including (Tumor Necrosis Factor- α (TNF-α), Interleukin-1 β (IL-1β), IL-6, IL-8, IL-12, IL-17 and the receptor activator of the factor nuclear kappa B ligand (RANK-L). Microorganisms located in the biofilm can reach the connective tissue and goes toward the alveolar bone, leading to the expression of RANK-L by osteoblasts, which can be accounted for the bone resorption seen during the disease process. If the infection fails to resolve, the release of pro-inflammatory mediators will continue and the activation of the B and T cells initiates the adaptive immunity. In this stage, the connective tissue become infiltrated by lymphocytes with predominantly more B cells (RANK-L) than T cells. The T cells will produce TNF-α, RANK-L and IL-17 which lead to increased osteoclastogenesis and bone resorption. This will result in the clinical signs of the disease characterized by increased clinical attachment loss (CAL.).