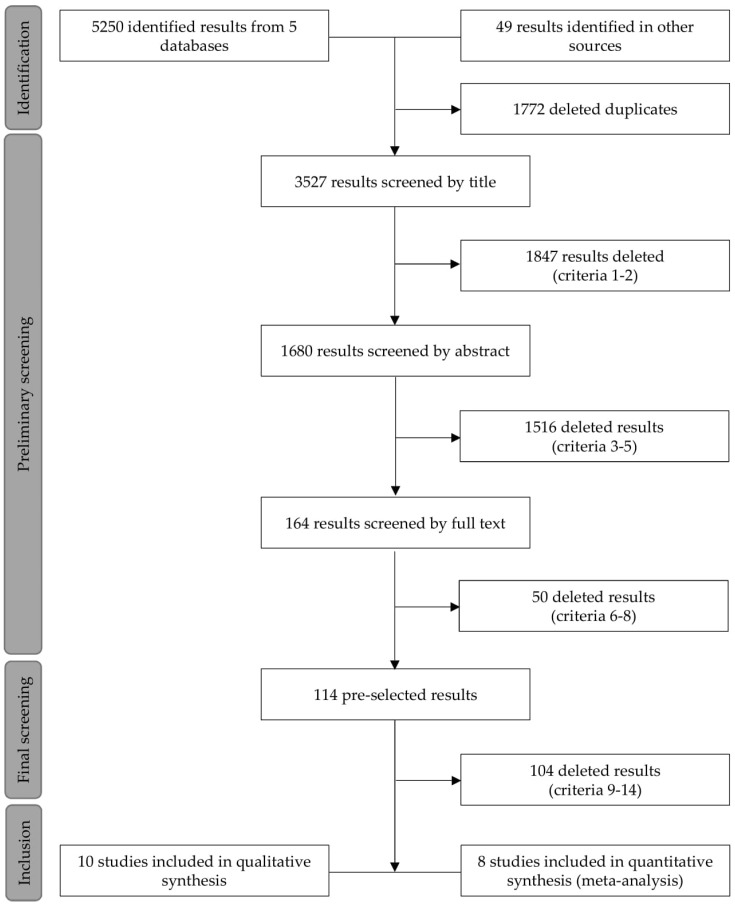
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) information flowchart for the selection and screening of primary studies. (1) Randomized clinical trial; (2) English language; (3) healthy individuals; (4) adults between 18 and 60 years old; (5) non-sedentary; (6) intervention of isolated, concentrated, and/or hydrolyzed whey protein; (7) intervention of physical activity of strength and/or resistance; (8) total interchange of participants between the groups for cross-over studies and sufficient time between the rounds of the experiment; (9) analysis of body composition between lean, fat-free and fat mass; (10) analysis of body composition by gold standard methods; (11) whey protein intervention, without associations, compared to placebos; (12) comparative group equated to whey protein intervention for calorie; (13) absence of variables parallel to listed interventions; and (14) non-inclusion of multiple publications.