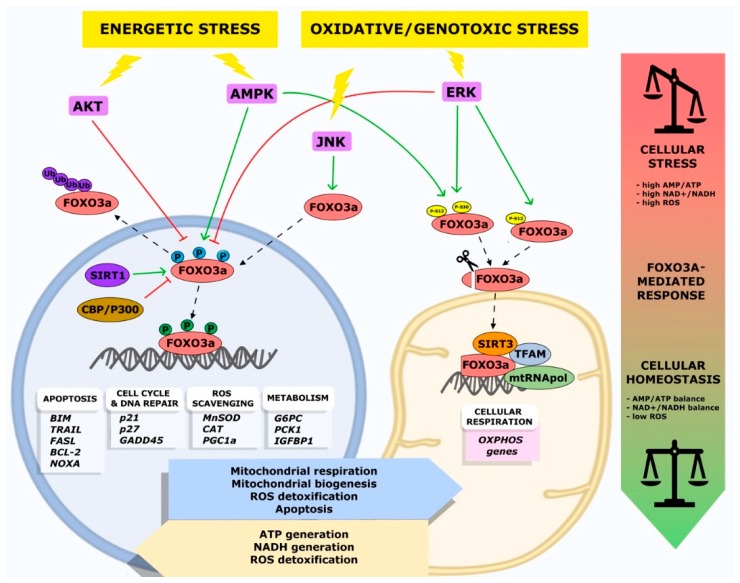
Figure 2.
Schematic representation of Forkhead box O3 (FOXO3a)-mediated stress response. Perturbations of cellular homeostasis, such as nutrient shortage, high concentration of intracellular ROS, or genotoxic stress, activate FOXO3a upstream stress sensors (purple boxes), which in turn modulate FOXO3a subcellular localization and/or activity through various post-translational modifications (PTMs) (green arrows represent activation signals, red bar-headed lines represent inhibitory effects). In the cytoplasm, FOXO3a is inactive and is targeted for poly-ubiquitination, which leads to its further proteasomal degradation. Upon phosphorylation by c-Jun N-terminal kinase (JNK), FOXO3a is shuttled into the nucleus, where its transcriptional activity is further regulated by an activator (e.g., 5′-AMP-activated protein kinase, AMPK; sirtuin 1, SIRT1) or repressor (e.g., AKT8 virus oncogene cellular homolog AKT, CREB binding protein and p300 (CBP/p300) signals. Depending on the PTM pattern, FOXO3a orchestrates different transcriptional programs involved in several cellular processes, including apoptosis, cell cycle progression, DNA repair, reactive oxygen species (ROS) detoxification, and cellular metabolism. Recent evidence showed that metabolic stress or chemotherapy treatment can also promote AMPK- and extracellular signal-regulated kinase (ERK) dependent mitochondrial accumulation of a FOXO3a cleaved form, which activates the expression of mitochondrial oxidative phosphorylation (OXPHOS) genes involved in cell survival. The crosstalk between FOXO3a nuclear and mitochondrial functions is crucial for the restoration and maintenance of cellular homeostasis.