Abstract
Introduction
Evidence indicates that menstrual cycle phase plays a role in smoking withdrawal symptoms and craving. Stress increases these symptoms. Whether the stress regulatory mechanism is associated with menstrual phase and withdrawal symptoms is not well understood.
Methods
Thirty-seven female smokers and 16 female nonsmokers were asked to complete a laboratory session. In each group, approximately half of the participants were tested when they were in the follicular phase and the other half was tested in the luteal phase. The session included resting baseline, stress, and recovery periods. Saliva samples for the measurement of cortisol and subjective measures of craving and withdrawal symptoms were collected at the end of each period.
Results
A series of repeated measures analysis of covariance found a significant smoking group × menstrual phase × sampling time interaction in cortisol levels (p < .05). Follow-up analyses indicated a reduced cortisol stress response in the luteal group relative to the follicular group in smokers (p < .02). This difference was not found in nonsmokers.
Conclusions
Menstrual cycle phase is related to hormonal stress response and smoking withdrawal symptomatology.
Implications
We show influences of the menstrual cycle phase on stress response among smokers. This is demonstrated by a reduced cortisol response to stress in the luteal group relative to the follicular group among smokers. This menstrual phase difference was not found in nonsmokers.
Introduction
Research to elucidate sex differences in smoking behavior is important for several reasons. Women have more difficulties in quitting smoking than men.1–6 Women report greater withdrawal-related negative affect and cue-related cravings for cigarettes than men.7–12 Psychosocial factors, such as perceived stress and negative affect, are associated with cessation outcomes in women relative to men.13
The menstrual cycle phase has been shown to be one potential mechanism of this sex difference (see refs.14–16 for extensive reviews). A meta-analysis of eight studies examining menstrual cycle phase differences (follicular vs. luteal) on self-report withdrawal symptoms found greater withdrawal scores during the luteal phase than the follicular phase.15 This same meta-analysis also found a trend in craving where craving scores were marginally (although it did not reach statistical significance; p = .06) elevated in the luteal phase relative to the follicular phase.15 Systematic reviews on studies examining the effects of the menstrual phase on magnitude of cigarette use, smoking topography, subjective effects, and smoking relapse have yielded mixed results.14,15 Variations in methodology, such as the identification of the menstrual cycle phase (via self-report, ovarian hormones) and questionnaires used to measure withdrawal symptoms, may account for differences.14–17 Inconsistent findings may also suggest the involvement of other biological mechanisms that are associated with smoking behavior and withdrawal symptoms and craving.
Stress is a well-known factor for maintenance of substance use, including smoking. Habitual smokers exhibit dysregulations in psychobiological stress response relative to nonsmokers.18–23 The hypothalamic-pituitary-adrenal (HPA) axis response to stress is associated with elevated withdrawal symptoms and craving22,24,25 and cessation failure.26–28 We recently reported sex differences in the stress response and smoking relapse29 where a reduced HPA stress response predicted early relapse in men, while greater HPA response was predictive of earlier relapse in women. These results suggest that the central stress regulatory system is associated with the severity of smoking withdrawal and risk for relapse.
Emerging evidence indicates a link exists between the menstrual cycle phase, HPA axis regulation, and craving.30–32 One study found that morning cortisol levels were negatively associated with nighttime craving among smokers who were tested in the luteal phase. However, this was not found among those in the follicular phase.30 Another study using a cross-over design found that, in the follicular phase, greater morning cortisol was associated with lower negative affect and withdrawal symptoms. In contrast, in the luteal phase, greater morning cortisol was related to reduced head rush and urges to smoke.31 Also using a cross-over design, another study tested effects of acute smoking withdrawal and menstrual cycle phase on cortisol and withdrawal symptoms.32 The results of this study found no menstrual phase cycle difference in cortisol variation, but there was an increase in craving during the first 24 hours of abstinence in the follicular phase while the level decreased in the luteal phase.32 These studies indicate that the association between diurnal cortisol variation and withdrawal symptomatology is modified by the menstrual cycle phase. Whether the menstrual phase influences hormonal responses to acute stress and withdrawal symptoms is largely unknown.
The purpose of the current study was to test the influence of smoking status (ad libitum smokers and nonsmokers) and menstrual cycle phase (follicular and luteal phases) on psychobiological responses to stress. We predicted that smokers tested during the luteal phase would exhibit an altered stress response relative to smokers tested during the follicular phase and nonsmokers.
Methods
Participants
Participants included in this study were part of a larger study to investigate predictors of stress and smoking relapse.22,29 Recruitment was completed through posting flyers and advertisements in the community. Participants interested in quitting smoking were invited to an on-site screening where eligibility was assessed using multiple self-report measures as described below. Participants needed to meet the following conditions to enroll in the study: no current or previous history of medical or psychiatric conditions; no active use of prescribed medications; body mass index between 18 and 30; social drinker (two or fewer drinks/day). Smokers must have smoked an average of 10 cigarettes/day for a minimum of 2 years and have a strong motivation for quitting as defined by 4/5 on a 1–5 rating scale of motivation to quit. Individuals who reported having irregular menstrual cycles (days since last menstruation started > 30), hysterectomies, oral contraceptive or hormonal intrauterine device usage, or menopause were not included in the present study. Eligible participants were scheduled for subsequent sessions where they signed a consent form and were compensated for their time. This study was approved by the Institutional Review Board of the University of Minnesota. A total of 37 smokers and 16 nonsmokers were enrolled in the current study.
Measures
At the beginning of each session, participants were asked to estimate the number of days since their most recent menstruation started and the regularity of their menstrual cycle. A backward counting method was used to define groups based on menstrual phase at the time of the session. (ie, follicular: 0–14 days; luteal: 15–30 days). Saliva samples were measured using Salivette tubes (Sarstedt, Rommelsdorf, Germany). Participants were instructed to place cotton rolls in their mouth until the roll became very saturated, and collected the rolls into a plastic tube. For cortisol assays, we used a time-resolved fluorescence immunoassay with a cortisol–biotin conjugate as a tracer,33 which had a sensitivity of 0.4 nmol/L as well as inter- and intra-assay coefficients of variation lower than 10% and 12%, respectively. MicroCO monitors (Micro Direct, Inc., Auburn, Maine) were used to evaluate carbon monoxide levels. The Minnesota Nicotine Withdrawal Scale (MNWS34) was administered to assess severity of withdrawal symptoms. The item “craving” was analyzed separately because of reports indicating conceptual differences in these two constructs.34 Positive affect and distress were assessed by the Revised Subjective States Questionnaire.35 Each item in this questionnaire used a 7-point rating scale with endpoints anchored by “Not at all” and “Very Strong.” Finally, demographic information, smoking history, nicotine dependence (Fagerström Test of Nicotine Dependence; FTND36), perceived stress (the Perceived Stress Scale; PSS37), and mood disturbances (the Profile of Mood State Questionnaire; POMS38) were also collected.
Procedure
Description regarding the protocol is detailed elsewhere.22,29 During the on-site screening, participants were asked to complete demographic, PSS, POMS, smoking history, and FTND forms after signing the consent. Participants were then scheduled for a laboratory session, which took place approximately 2 weeks after the screening. All the laboratory sessions were held between noon and 2 pm to control for diurnal changes in cortisol. Participants were instructed to abstain from caffeine for 4 hours and both alcohol and exercise for 24 hours before the laboratory session. Smokers smoked their preferred brand of cigarette immediately before the session to minimize nicotine withdrawal. Participants were tested individually. Upon arrival at the laboratory, the participant was greeted by the experimenter and was asked to sit on a comfortable chair. The participant was asked about days since last menstruation started as well as contraceptive use. A sample of saliva (the first sample) and expired carbon monoxide were then collected. This baseline period (approximately 45 minutes) was followed by a 20 minutes resting period, 20 minutes stress period (10 minutes of public speech and 10 minutes of mental arithmetic tests), and 20 minutes poststress recovery. A modified version of the Trier Social Stress Test39,40 was used to induce stress. The test has been validated40 and used in studies examining psychobiological stress response among smokers20 and stress response and smoking relapse.26,29 Saliva samples, as well as subjective measures, were collected at the end of each 20 minute period (ie, rest, stress, and recovery). Nonsmoking individuals were recruited and tested in parallel with the same laboratory protocol.
Data Analysis
The phase of the menstrual cycle was determined by self-reported days since last menstruation started (ie, follicular: 0–14 days; luteal: 15–30 days). As a result, 17 smokers were tested when they were in the follicular phase and 20 were tested during the luteal phase. Similarly, eight nonsmokers were tested during the follicular phase and eight were tested during the luteal phase. A chi-square test examining a link between smoking status and menstrual phase found no difference (χ2 < 1) indicating equal distribution of menstrual phase groups across smoking groups.
Salivary cortisol data were log transformed to meet the normality assumption. Analysis on baseline measures (described below) found a significant difference in years of smoking between follicular and luteal group smokers. Thus, years of smoking was included as a covariate in the following tests. The number “0” identified nonsmokers in these models. A series of 2 smoking (smoker, nonsmoker) × 2 menstrual phase (follicular, luteal) × 3 time (rest, stress, recovery) repeated measures analysis of covariance with Greenhouse-Geisser correction were conducted to examine cortisol, positive affect, and distress. A series of 2 menstrual phase (follicular, luteal) × 3 time (rest, stress, recovery) repeated measures analysis of covariances controlling for years of smoking were conducted to test craving and withdrawal symptoms. Finally, 2 smoking (smoker, nonsmoker) × 2 phase (follicular, luteal) analyses of variance were conducted to test demographic variables, the first sample cortisol, PSS, and POMS. Smoking history variables were examined using one-way analyses of variances including menstrual phase (follicular, luteal) as a between subject factor.
Results
The mean age of the present sample was 31.7 years (standard deviation [SD]: ± 11.7), body mass index was 24.1 (SD: ± 3.5), and average hours of nightly sleep over the previous week was 7.1 hours (SD: ± 1.0; Table 1). There was no group, phase, nor group × phase effects in these demographic variables nor were there effects for PSS, POMS, or the first sample saliva cortisol. Smokers had fewer years of education than nonsmokers [F(1, 48) = 4.88, p = .03, η2 = 0.09]. On average, smokers smoked 16.7 cigarettes/day (SD: ± 5.8) had an average FTND score of 5.04 (SD: ± 2.1), and carbon monoxide level of 24.6 parts per million (SD: ±15.0). These variables did not differ across menstrual phase groups. Smokers who were in the luteal phase had longer history (in years) of cigarette use than those in the follicular phase [F(1, 34) = 6.57, p = .02, η2 = 0.16].
Table 1.
Sample Characteristics
| Nonsmokers (n = 16) | Smokers (n = 37) | |||
|---|---|---|---|---|
| Follicular (n = 8) | Luteal (n = 8) | Follicular (n = 17) | Luteal (n = 20) | |
| Age (years) | 28.9 (4.1) | 34.8 (4.1) | 28.4 (2.8) | 34.5 (2.6) |
| Body mass index | 24.4 (1.3) | 23.2 (1.3) | 23.4 (0.9) | 24.9 (0.8) |
| Education (years)a,* | 15.9 (0.9) | 15.9 (0.9) | 13.9 (0.7) | 14.4 (0.6) |
| Sleep (average hours) | 7.3 (0.3) | 6.8 (0.3) | 7.3 (0.2) | 7.0 (0.2) |
| Cortisol first sample (nmol/L)b | 1.9 (0.2) | 1.6 (0.2) | 2.1 (0.2) | 2.0 (0.1) |
| Mood disturbancec | 12.9 (11.0) | 17.3 (11.0) | 30.5 (7.5) | 27.6 (7.0) |
| Perceived stressd | 18.1 (2.0) | 17.4 (2.0) | 20.6 (1.4) | 19.9 (1.3) |
| Cigarettes (per day) | n/a | n/a | 15.6 (1.5) | 17.6 (1.3) |
| Duration (years)a,* | n/a | n/a | 5.0 (1.9) | 11.7 (1.7) |
| Nicotine dependencee | n/a | n/a | 5.0 (0.5) | 5.1 (0.5) |
| Cotinine (ng/mL) | n/a | n/a | 171.2 (47.6) | 169.4 (43.2) |
| CO (ppm) | n/a | n/a | 22.2 (3.7) | 26.8 (3.5) |
Entries show mean and standard deviation of the mean. CO, carbon monoxide.
aThere was a significant main effect of smoking group.
bLog transformation was applied to cortisol levels.
cThis was assessed by the Profile Of Mood State Questionnaire.
dThis was assessed by the Perceived Stress Scale.
eThis was assessed by the Fagerström Test of Nicotine Dependence.
*p < .05.
A significant time effect in positive affect [F(2, 94) = 15.7, p < .001, η2 = 0.25] indicated decreased levels in response to stress, and a time effect in distress [F(2, 94) = 4.90, p = .009, η2 = 0.09] reflected increased levels immediately after stress (Table 2). A marginally significant menstrual phase × time interaction in distress [F(2, 94) = 2.83, p = .06, η2 = 0.06] suggested a trend toward greater increase in response to stress in the luteal group than the follicular group. Self-reported craving [F(2, 66) = 3.69, p = .03, η2 = 0.10] and withdrawal symptoms [F(2, 66) = 3.82, p = .03, η2 = 0.10] increased in response to stress as indicated by main effects of time.
Table 2.
Self-report Positive Affect, Distress, Craving for Cigarettes, and Withdrawal Symptoms
| Nonsmokers (n = 16) | Smokers (n = 37) | |||
|---|---|---|---|---|
| Follicular (n = 8) | Luteal (n = 8) | Follicular (n = 17) | Luteal (n = 20) | |
| Positive affecta | ||||
| Rest | 21.8 (3.0) | 16.2 (3.0) | 15.3 (2.0) | 15.8 (2.0) |
| Stress | 15.6 (2.8) | 10.5 (2.8) | 10.4 (1.8) | 10.7 (1.9) |
| Recovery | 19.7 (3.0) | 15.3 (3.0) | 15.5 (2.0) | 16.4 (2.0) |
| Distressa | ||||
| Rest | 2.8 (1.9) | 5.2 (1.9) | 5.6 (1.2) | 4.6 (1.3) |
| Stress | 3.0 (2.2) | 8.8 (2.2) | 6.7 (1.5) | 7.6 (1.5) |
| Recovery | 3.4 (2.0) | 4.3 (2.0) | 5.1 (1.3) | 4.5 (1.4) |
| Cravinga | ||||
| Rest | n/a | n/a | 2.5 (0.4) | 2.1 (0.3) |
| Stress | n/a | n/a | 3.8 (0.6) | 3.3 (0.5) |
| Recovery | n/a | n/a | 2.3 (0.6) | 3.0 (0.5) |
| Withdrawal symptomsa | ||||
| Rest | n/a | n/a | 7.5 (1.9) | 5.0 (1.7) |
| Stress | n/a | n/a | 10.9 (2.0) | 10.5 (1.8) |
| Recovery | n/a | n/a | 7.6 (1.7) | 5.7 (1.5) |
Entries show mean and standard deviation of the mean adjusting for years of smoking.
aMain effect of time was significant.
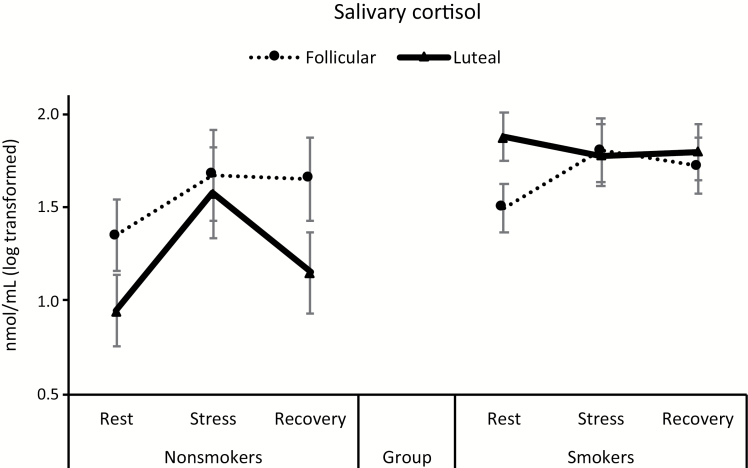
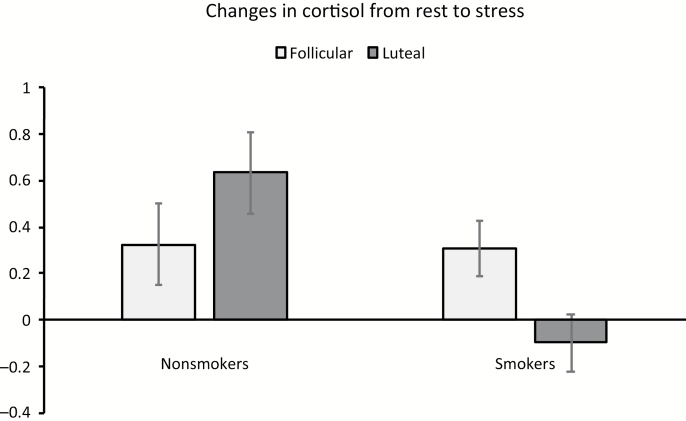
For salivary cortisol, there was a significant main effect of time [F(2, 92) = 6.85, p = .002, η2 = 0.13]. This was qualified by a group × time interaction [F(2, 92) = 3.12, p = .049, η2 = 0.06] indicating a flattened stress response pattern in smokers relative to nonsmokers. In addition, a significant smoking × menstrual phase × time interaction in cortisol was found [F(2, 92) = 3.88, p = .02, η2 = 0.08; Figure 1]. As a follow-up, a change score analysis was conducted (the value during stress period was subtracted from the value during the resting baseline). A 2 group × 2 phase analysis of covariance found a significant group × phase interaction [F(1, 46) = 5.92, p = .02, η2 = 0.11], indicating lower cortisol response in the luteal group than in the follicular group among smokers (p = .03). In contrast, this phase difference was not observed among nonsmokers (p = .19; Figure 2).
Figure 1.
Changes in salivary cortisol in response to acute stress. Entries indicate mean and bars indicate standard error of the mean.
Figure 2.
Smoking group and menstrual phase differences in stress response. Entries indicate mean and bars indicate standard error of the mean.
Discussion
The current study demonstrated, for the first time, that salivary cortisol responses to acute laboratory stress were reduced in smokers who were tested in the luteal phase. Increases in distress levels in response to stress tended to be greater during the luteal phase than the follicular phase regardless of the smoking status. Craving and withdrawal symptoms increased in response to stress among smokers. The current findings are consistent with our hypothesis that the menstrual cycle moderates the link between HPA stress response, craving, and withdrawal symptoms.
The current findings have several implications. The smoking group × time interaction indicative of attenuated cortisol stress response in smokers relative to nonsmokers is consistent with previous research.18,20 The current results further expand this observation by showing that the reduced stress response was pronounced in smokers who were in their luteal phase. Recent evidence indicates that smoking behavior is expected to decrease in the luteal phase. This theory was formulated based on animal studies directly linking estrogen to the rewarding properties of drugs,41 preclinical studies demonstrating protective effects of progesterone on nicotine addiction,42 and cross-sectional evidence of high progesterone levels during the luteal phase.16 Our results were not in agreement with this view but they are consistent with studies showing associations between blunted HPA stress response, elevated craving, and early smoking relapse.26 It is possible that the use of self-report in identifying the menstrual phase influenced our results. It is also plausible that acute stress (or production of stress hormones) influenced the effects of ovarian hormones on subjective craving. Elucidating the role of stress on ovarian hormones should improve our understanding of psychobiological mechanisms of stress and smoking (smoking behavior, withdrawal symptomatology, and smoking abstinence) among females.
This study does have a few limitations. The sample size was small and the use of cross-sectional design is limiting. A counterbalanced design in which the menstrual cycle phase of the first session is randomized and the same individual is tested during each phase of menstrual cycle may improve results. The use of dichotomous classification of menstrual phase cycle (0–14 vs. 15–30 days) based on self-report may also have limited our results because not all women fall neatly into this classification due to variability in the month-to-month timing of ovulation. This is especially important as the late luteal phase is hormonally different from the early luteal phase and minor errors in recall may affect this estimate.16 Some studies have tested women by further separating early from late follicular and luteal phases.43 Also, the use of hormonal verification may be useful in clarifying the role of menstrual cycle on stress. Finally, future research should examine individuals with a history of premenstrual dysphoric disorder as well as those who have had menopause or hysterectomy to examine whether these conditions are associated with stress and craving. The strength of this study was that it measured both subjective and objective measures, used careful methodology to induce stress, and included nonsmoking individuals to assess the role of cortisol stress response in the link among smoking, menstrual phase, and stress.
In conclusion, this study found that habitual smoking during the luteal phase was associated with attenuated cortisol responses to stress, suggesting the important role of menstrual cycle phase in psychobiological stress response as well as in craving and in withdrawal symptoms.
Funding
This research was supported in part by the National Institute of Health (R01DA016351 and R01DA027232 to MN).
Declaration of Interests
None declared.
Acknowledgments
We thank Angie Forsberg, Elizabeth Ford, and Barbara Gay for assistance with data collection and management.
References
- 1. Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15(5):391–411. [DOI] [PubMed] [Google Scholar]
- 2. Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10(7):1245–1250. [DOI] [PubMed] [Google Scholar]
- 3. Piper ME, Cook JW, Schlam TR, et al. . Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine Tob Res. 2010;12(6):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67(4):555–562. [DOI] [PubMed] [Google Scholar]
- 5. Smith PH, Kasza KA, Hyland A, et al. . Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res. 2015;17(4):463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99(11):1462–1469. [DOI] [PubMed] [Google Scholar]
- 7. Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Exp Clin Psychopharmacol. 2007;15(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Exp Clin Psychopharmacol. 2013;21(4):269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Exp Clin Psychopharmacol. 2003;11(4):276–285. [DOI] [PubMed] [Google Scholar]
- 10. Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78(3):647–652. [DOI] [PubMed] [Google Scholar]
- 11. Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23(2):209–224. [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Azizian A, Monterosso J, et al. . Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10(11):1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakajima M, al’Absi M. Predictors of risk for smoking relapse in men and women: a prospective examination. Psychol Addict Behav. 2012;26(3):633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res. 2006;8(5):627–638. [DOI] [PubMed] [Google Scholar]
- 15. Weinberger AH, Smith PH, Allen SS, et al. . Systematic and meta-analytic review of research examining the impact of menstrual cycle phase and ovarian hormones on smoking and cessation. Nicotine Tob Res. 2015;17(4):407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wetherill RR, Franklin TR, Allen SS. Ovarian hormones, menstrual cycle phase, and smoking: a review with recommendations for future studies. Curr Addict Rep. 2016;3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allen AM, McRae-Clark AL, Carlson S, et al. . Determining menstrual phase in human biobehavioral research: a review with recommendations. Exp Clin Psychopharmacol. 2016;24(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roy MP, Steptoe A, Kirschbaum C. Association between smoking status and cardiovascular and cortisol stress responsivity in healthy young men. Int J Behav Med. 1994;1(3):264–283. [DOI] [PubMed] [Google Scholar]
- 19. Straneva P, Hinderliter A, Wells E, Lenahan H, Girdler S. Smoking, oral contraceptives, and cardiovascular reactivity to stress. Obstet Gynecol. 2000;95(1):78–83. [DOI] [PubMed] [Google Scholar]
- 20. al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74(2): 401–410. [DOI] [PubMed] [Google Scholar]
- 21. Kirschbaum C, Strasburger CJ, Langkrär J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993;44(3):527–531. [DOI] [PubMed] [Google Scholar]
- 22. al’Absi M, Nakajima M, Grabowski J. Stress response dysregulation and stress-induced analgesia in nicotine dependent men and women. Biol Psychol. 2013;93(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girdler SS, Jamner LD, Jarvik M, Soles JR, Shapiro D. Smoking status and nicotine administration differentially modify hemodynamic stress reactivity in men and women. Psychosom Med. 1997;59(3):294–306. [DOI] [PubMed] [Google Scholar]
- 24. Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24(2):247–255. [DOI] [PubMed] [Google Scholar]
- 25. McKee SA, Sinha R, Weinberger AH, et al. . Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25(4):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl). 2005;181(1):107–117. [DOI] [PubMed] [Google Scholar]
- 27. Ussher M, West R, Evans P, et al. . Reduction in cortisol after smoking cessation among users of nicotine patches. Psychosom Med. 2006;68(2): 299–306. [DOI] [PubMed] [Google Scholar]
- 28. Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry. 1998;43(7):525–530. [DOI] [PubMed] [Google Scholar]
- 29. al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine Tob Res. 2015;17(4):382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen AM, Allen SS, Widenmier J, Al’absi M. Patterns of cortisol and craving by menstrual phase in women attempting to quit smoking. Addict Behav. 2009;34(8):632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huttlin EA, Allen AM, Tosun NL, Allen SS, al’Absi M. Associations between adrenocortical activity and nicotine response in female smokers by menstrual phase. Addict Behav. 2015;50:135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carlson SC, Allen AM, Allen SS, al’Absi M. Differences in mood and cortisol by menstrual phase during acute smoking abstinence: a within-subject comparison. Exp Clin Psychopharmacol. 2017;25(5):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43(7):683–692. [DOI] [PubMed] [Google Scholar]
- 34. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 35. Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. J Psychosom Res. 1980;24(3–4):125–130. [DOI] [PubMed] [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 37. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 38. McNair DM, Lorr M, Droppleman LF.. POMS Manuel-Profile of Mood Questionnaire. San Diego: Edits; 1992. [Google Scholar]
- 39. Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. [DOI] [PubMed] [Google Scholar]
- 40. Al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34(3): 266–275. [DOI] [PubMed] [Google Scholar]
- 41. Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–279. [DOI] [PubMed] [Google Scholar]
- 42. Lynch WJ, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordon JL, Girdler SS. Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology. 2014;51(4):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]