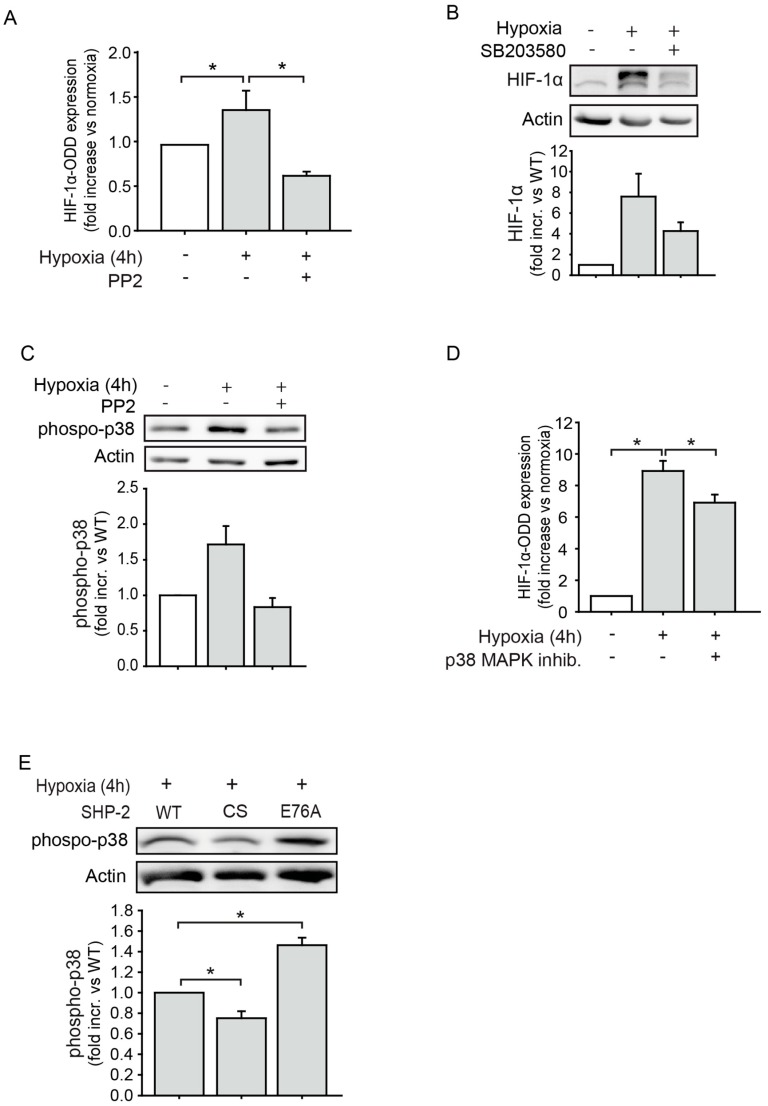
Figure 3.
The proteasomal HIF-1α degradation is dependent on Src kinase and p38 mitogen-activated protein kinase (MAPK) signaling. (A) Whereas hypoxia inhibited 26S proteasome activity in endothelial cells, as seen by increased expression of HIF1-ODD-Luc, inhibition of Src kinase (PP2, 100 nM) reversed this (* p < 0.05; n = 9). (B) Inhibition of p38 MAPK (SB203580, 10 µM) in endothelial cells impaired hypoxia induced HIF-1α expression (n = 6). (C) Src kinase inhibition (PP2, 100 nM) reduced hypoxia induced p38 MAPK activation (n = 2). (D) p38 MAPK inhibition (SB203580, 10 µM) increased 26S proteasome activity, as measured by a lower level of HIF1-ODD-Luc reporter expression (* p < 0.05; n = 4). (E) Expression of dominant negative SHP-2 (CS) impaired hypoxia induced p38 MAPK phosphorylation, whereas expression of constitutively active SHP-2 (E76A) enhanced this compared to SHP-2 WT (* p < 0.05; n = 4). Graphs underneath blots show the protein band densities normalized to β-actin.