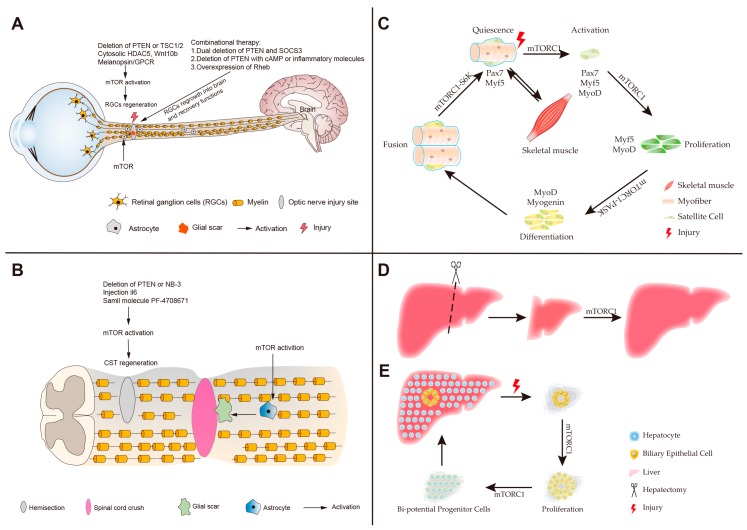
Figure 3.
The roles of mTOR in the regeneration of neurons, muscles and the liver. (A) The optic nerve injury model. Activation of mTOR by deleting phosphatase and tensin homolog (PTEN) or tuberous sclerosis complex 1/2 (TSC1/2), the upstream cytosolic HDAC5 (histone deacetylase 5), Wnt10b (wingless-type MMTV integration site family, member 10b), and melanopsin/GPCR (cell-type-specific G protein-coupled receptor) robustly enhances the regeneration of retinal ganglion cells (RGCs). Combinational therapies augment the RGCs long-distance regeneration for visual function recovery through overexpression of Ras homolog enriched in brain (Rheb); the dual deletion of PTEN and suppressor of cytokine signaling 3 (SOCS3); deficiency of PTEN combined with injecting of cyclic adenosine monophosphate (cAMP) or inflammatory molecules (oncomodulin or zymosan). However, the excessive mTOR activation of astrocytes contributes to forming glial scar to inhibit axonal regeneration. (B) The spinal cord injury (SCI) model. In spinal cord crush model, the activated mTOR of astrocytes facilitates glial scar formation resulting in impeding the spinal cord regeneration after SCI. In the hemisection model, the stimulation of mTOR promotes the corticospinal tract (CST) regeneration post-SCI. (C) A schematic representation of skeletal muscle regeneration. mTORC1 stimulates satellite cells activation and proliferation, and their progenies differentiate into myoblasts under mTORC1 regulation. mTORC1 also promotes the fusion of myoblasts to form myofibers. (D) Partial hepatectomy (PH) model. Liver regeneration after PH is via the self-replication of existing hepatocytes, and mTORC1 regulates hepatocyte proliferation. (E) The severe liver injury model. Liver regeneration is via the trans-differentiation of cholangiocytes. In the process, mTORC1 regulates the proliferation of cholangiocytes and the formation of Bi-potential Progenitor Cells.