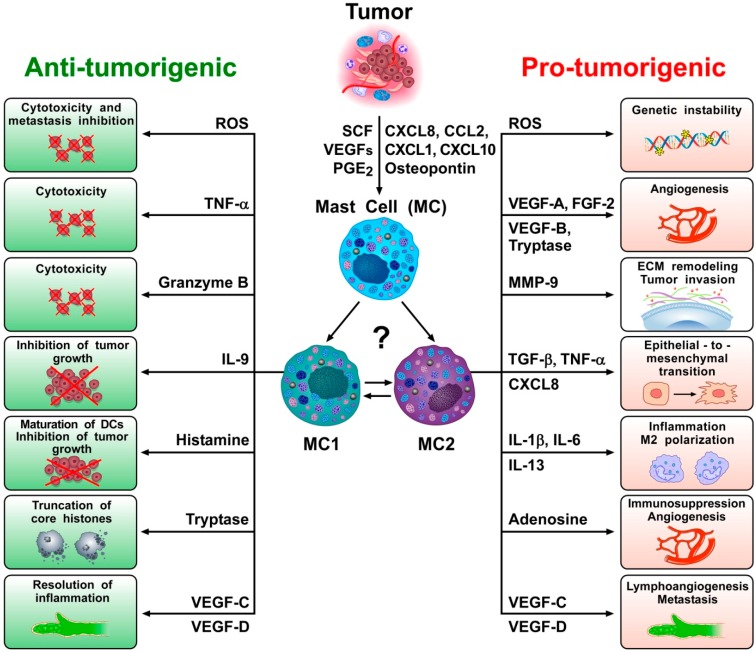
Figure 1.
Mast cells can be recruited into tumor microenvironments (TMEs) by several chemotactic molecules (e.g., SCF, VEGFs, PGE2, CXCL8, CCL2, CXCL1, CXCL10, osteopontin) produced by tumor or immune cells [49]. However, mast cells in the TME can exert anti-tumorigenic and/or pro-tumorigenic roles. Similarly to neutrophils (N1 and N2) and macrophages (M1 and M2), it is possible that the complex biochemical milieu of the TME (and of tumor cells themselves) can polarize mast cells toward anti-tumorigenic MC1 or pro-tumorigenic MC2 mast cell types. Reactive oxygen species (ROS) are chemically reactive free radicals that potentially function as a double-edged sword [54]. Rodent and human mast cells can produce functionally active ROS [55] and excessive ROS may induce cytotoxic effects that can contribute to tumor regression. Mast cells also can exert direct tumor cytotoxic effects via TNF-α [56,57,58,59] and/or granzyme B [60,61]. IL-9 produced by mast cells can inhibit tumor cell engraftement [62]. Histamine promotes dendritic cell (DC) maturation and inhibits tumor growth [63,64]. Tryptase can be taken up into the nucleus of human melanoma cells causing truncation of histones and inhibition of cell proliferation [65]. Human mast cells also can release lymphangiogenic factors (VEGF-C and VEGF-D) [45,66], and increasing evidence indicates that lymphangiogenesis can play an active role in the resolution of inflammation [67,68]. However, the presence of large amounts of ROS can outstrip the capacity of cellular DNA repair systems, triggering genomic instability and transcription errors that may foster tumor initiation [69]. Mast cells also represent a potentially major source of several angiogenic molecules (VEGF-A, VEGF-B, FGF-2, tryptase) [45,70,71,72,73,74]. In addition, MMP-9 can induce degradation of the extracellular matrix, leading to cancer cell invasion and metastasis [75]. TGF-β, CXCL8 and TNF-α can induce epithelial-to-mesenchymal transition [48,76]. Proinflammatory cytokines such as IL-1β [49,77,78,79,80,81] and IL-6 [82,83] can contribute to chronic inflammation in tumor microenvironment. IL-13 favors M2 polarization of tumor-associated macrophages [77]. Adenosine can be released by activated mast cells and potentiates the release of angiogenic and lymphangiogenic factors from human mast cells [45]. VEGF-C and VEGF-D are the major lymphangiogenic factors produced by human mast cells and can contribute to the formation of metastasis [84,85].