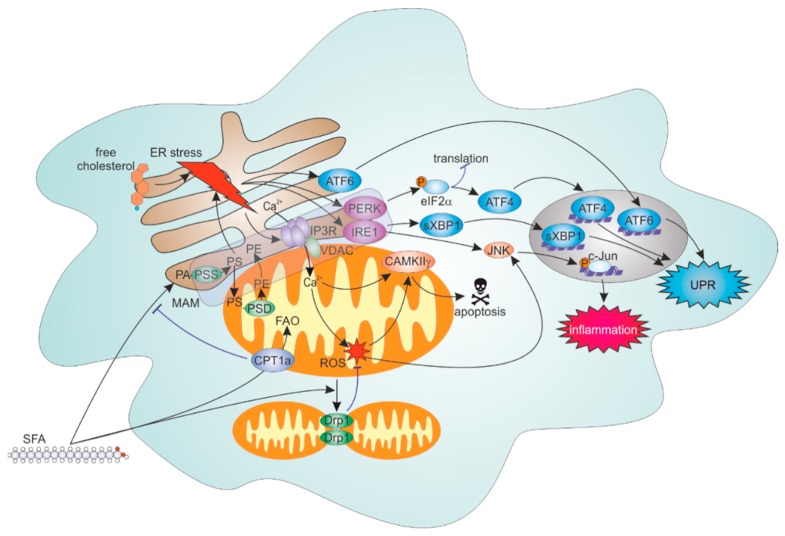
Figure 1.
Endoplasmic reticulum (ER)-mitochondrial regulation of lipid overload-induced stress responses. Excessive incorporation of saturated fatty acids (SFA) into ER phospholipids or accumulation of free cholesterol in the ER induce ER stress response mediated by activating transcription factor 6 (ATF6), protein kinase RNA-activated (PKR)-like ER kinase (PERK) and inositol requiring enzyme 1 (IRE1) sensor proteins. Concomitantly, SFA undergo fatty acid β-oxidation (FAO), which attenuates SFA incorporation into phosphatidylserine (PS) and phosphatidylethanolamine (PE) and dampens ER stress. Furthermore, fatty acids induce Drp1-dependent mitochondrial fragmentation, which attenuates mitochondrial ROS formation and activation of pro-inflammatory c-Jun N-terminal kinase (JNK) signaling. ER stress also provokes inositol triphosphate receptors (IP3R) activation and mitochondrial Ca2+ overload. Under ER stress conditions, Ca2+/calmodulin-dependent protein kinase IIγ (CAMKIIγ) may translocate to mitochondria and undergo Ca2+- and ROS-dependent activation, promoting apoptosis. CPT: Carnitine palmitoyltransferase; PA: Phosphatidic acid; PSS: Phosphatidylserine synthase; PSD: Phosphatidylserine decarboxylase.