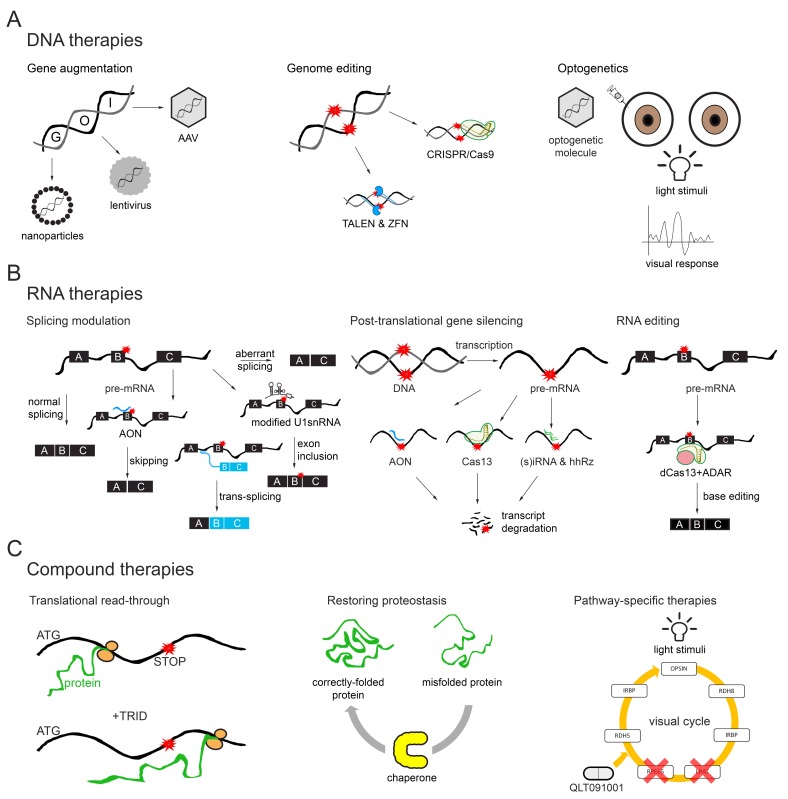
Figure 2.
Schematic and simplified representation of the several types of molecular therapies. (A) DNA therapies are represented by gene augmentation, genome editing and optogenetics. In gene augmentation, the entire coding sequence of the gene of interest (GOI) is delivered using different vectors. Genome editing employs nucleases able to edit the DNA at a specific position; CRISPR/Cas9 is depicted in green, guide RNA in dark yellow and ZFN and TALEN in blue. Mutations are depicted in red. In optogenetics, a light-sensitive molecule is delivered to the eye to give photosensitive properties to remaining retinal neurons. (B) RNA therapies; splicing modulation can be achieved using AONs (in dark blue) for (pseudo)exon skipping or modified U1 snRNA (in black) to favour exon inclusion in cases where mutations (in red) are found in the donor splice site. Trans-splicing occurs between two independent RNA molecules—The original transcript and the exogenous molecule without the mutation. In all cases, splicing is modulated to obtain a transcript with full or residual function. Post-translational gene silencing can be achieved by degrading the RNA transcript using AONs (dark blue), Cas13 (green) with a guide RNA (dark yellow) or (s)iRNA and hhRz (in green). These approaches can be used for dominant-negative mutations by promoting allele-specific degradation. Mutations are indicated in red. With RNA editing using CRISPR/Cas technology, dead Cas13 (in green) is conjugated with an adenosine deaminase (dark yellow) acting at the RNA level (ADAR, in light red). This molecule is guided to the mutation using a guide RNA binding on top of the mutation (in red) to induce a G-to-A transversion. (C) Representative examples of compound therapies. Translational read-through allows the ribosome to continue protein synthesis despite a premature stop codon (in red) in the presence of the translational read-through-inducing drugs (TRIDs). Restoring proteostasis can be accomplished by using chaperones to properly fold proteins. QLT091001 is a pathway-specific therapy that acts in the visual cycle and can be used when for example, RPE65 or LRAT are mutated.