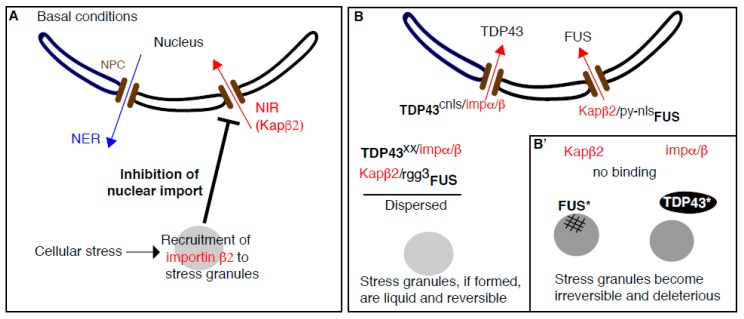
Figure 2.
The complex relationship between stress granules, cyto-nuclear transport and ALS. (A): In basal growth conditions, the nuclear import receptor (NIR) Karyopherin β2 (Kapβ2) associates to its cognate cargoes and imports them to the nucleus through the nuclear pore complex (brown bars on the nuclear envelope). Upon cellular stress, Kapβ2 is recruited to stress granules and is no longer available to function as a receptor. As a result, stress granule formation triggered by cellular stress causes inhibition of cyto-nuclear transport. (B): The RNA-binding protein FUS and TDP43 are normally imported in the nucleus by binding to their cognate NIRs (Kapβ2 and Impα/β, respectively). The pool of FUS and TDP43 that is present in the cytoplasm remains dispersed through their binding to Kapβ2 and Impα/β, respectively but via other domains (such as FUS-rgg3). (B’): Upon cellular stress and/or expression of mutated FUS and TDP43 that prevent their binding to their NIRs, mutated FUS (FUS*) and TDP43 (TDP43*) aggregates in a deleterious manner but in two different ways. FUS* is recruited to stress granules and aggregates within, leading stress granules to be become solid and irreversible. On the other hand, TDP43* aggregates on its own next to stress granules and negatively impact their dynamics via a mechanism that is still to be elucidated.