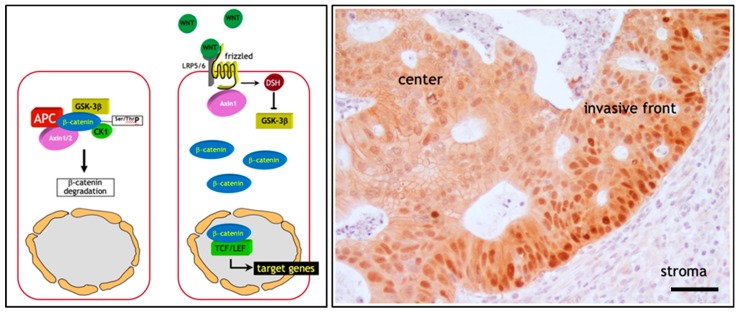
Figure 1.
The (a) Wnt/β-catenin signal transduction pathway and the (b) β-catenin paradox in colon cancer. (a) Illustration of the canonical Wnt signaling in homeostasis. Left panel: In the absence of Wnt ligands, intracellular β-catenin levels are controlled by a destruction complex encompassing protein phosphatase 2A (PP2a), glycogen synthase kinase 3 (GSK3β) and casein kinase 1α (CK1α), adenomatous polyposis coli (APC), and Axin1/2. This complex binds and phosphorylates β-catenin at serine and threonine residues, thereby targeting it for ubiquitination and proteolytic degradation by the proteasome. Right panel: In presence of Wnt, co-activation of the Frizzled and LRP5/6 (low-density lipoprotein receptor-related proteins) receptors prevents the formation of the destruction complex leading to the stabilization and consequent translocation of β-catenin from the cytoplasm to the nucleus. Here, β-catenin interacts with members of the TCF/LEF family of transcription factors and modulates the expression of a broad spectrum of Wnt downstream target genes. Adapted from [24]. (b) The β-catenin paradox in colon cancer. β-catenin IHC analysis of the invasive front of a colon carcinoma show marked nuclear β-catenin accumulation in the proximity of the stromal microenvironment. In contrast, the majority of tumor cells localized inside the tumor mass are characterized by membrane-bound and cytoplasmic β-catenin staining. Scale bar: 50 µm.