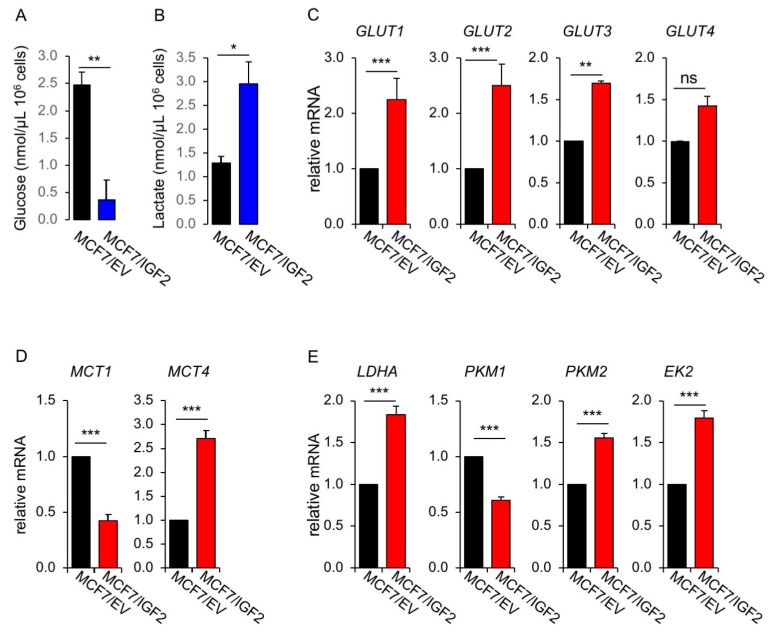
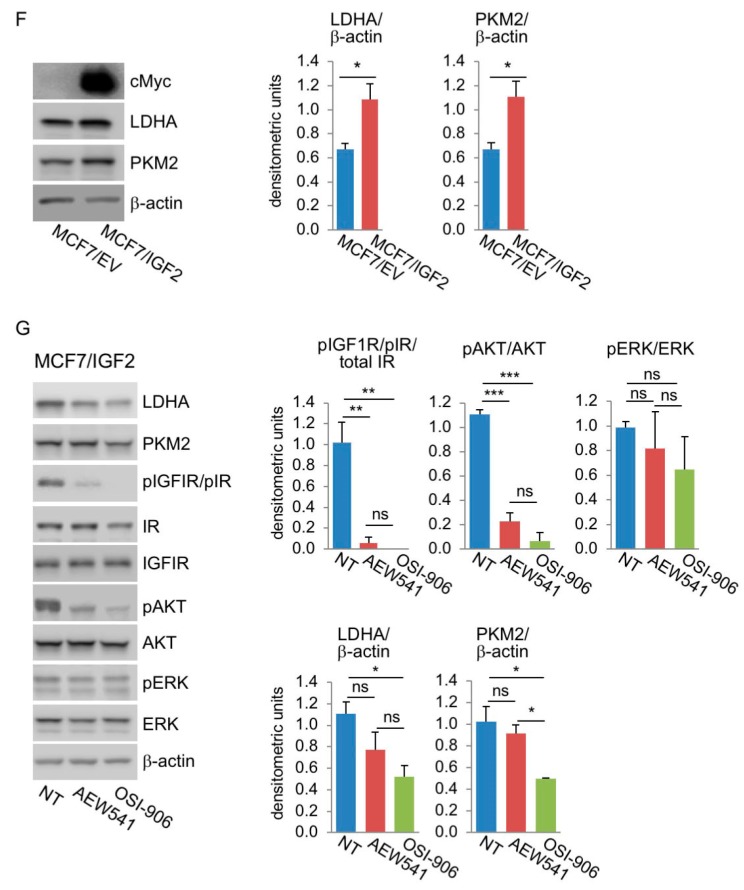
Figure 2.
Glucose consumption, lactate production and expression of glucose and lactate transporters and glycolytic enzymes. Glucose (A) and lactate (B) concentrations were measured in media conditioned from MCF7/IGF2 and MCF7/EV control cells after 48 h using colorimetric assays (see Methods). Values are mean ± SE of three separate experiments (** p < 0.01; * p < 0.05). Cells were processed to evaluate mRNA expression for (C) glucose transporters (GLUT)1–4, (D) monocarboxylate transporter (MCT)1 and MCT4, (E) glycolytic enzymes lactate dehydrogenase A (LDHA), pyruvate kinase M1 (PKM1), pyruvate kinase M2 (PKM2), hexokinase-2 (EK2), by qRT-PCR analysis. MCF7/EV cells were used as control and GAPDH used as the housekeeping control gene. Values are means ± SEM of three separate experiments. (F) MCF7/IGF2 cells and the corresponding control cells (MCF7/EV) were grown in medium containing 10% charcoal stripped- fetal bovine serum (FBS) for 48 h. Cells were then lysed, analyzed by SDS-PAGE and immunoblotted with the indicated primary antibodies to evaluate the expression of glycolytic enzymes LDHA and PKM2. A representative blot of three independent experiments is shown. Graphs represent the mean ± SEM of densitometric analysis of three independent experiments, where LDHA and PKM2 were normalized to β-actin. (G) LDHA, PKM2, pIGF1R/pIR, IR, IGF1R, pAKT, AKT, pERK and ERK were then evaluated in MCF7/IGF2 cells before and after exposure to two different IGF1R/IR tyrosine-kinase inhibitors (TKI) (a pyrrolo(2,3-d) pyrimidine derivative, NVP-AEW541, and a selective and orally bioavailable dual IGF1R/IR inhibitor, OSI-906) at the concentration of 0.5 µM every 24 h, for 48 h. β-actin was used to control for protein loading. Blots are representative of three independent experiments. Graphs represent the mean ± SEM of densitometric analysis of three independent experiments.