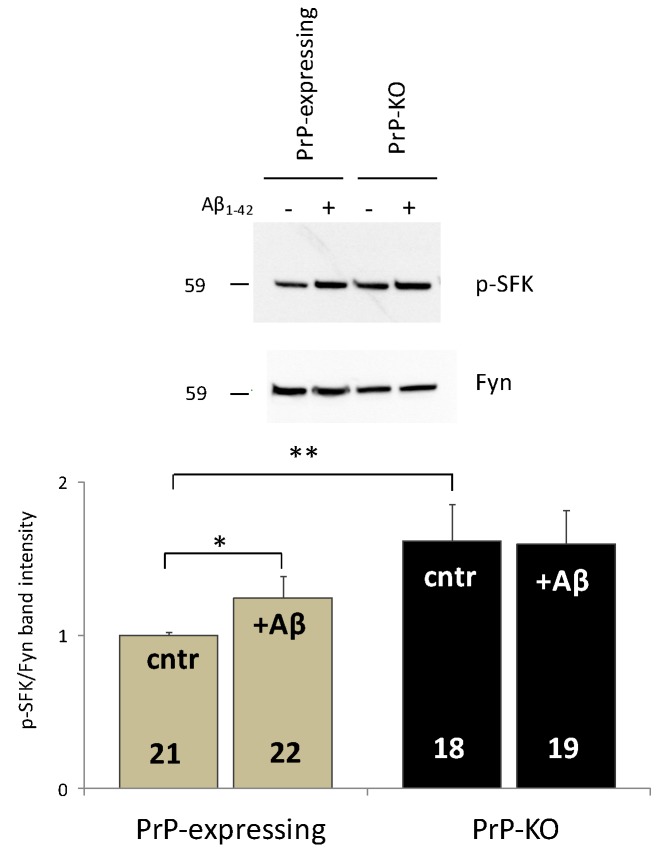
Figure 1.
Soluble Aβ1–42 oligomers abrogate the PrP-dependent control over Fyn in PrP-expressing primary cortical neurons. Both the representative western blot (WB) obtained after probing PrP-expressing and PrP-KO cortical neurons (treated (+), or not (−), with Aβ1–42 oligomers) with antibody to active (auto-phosphorylated, p) SFK or total Fyn (upper panel), and the densitometric analysis of the pSFK immuno-reactive bands normalized to the corresponding signal of total Fyn (lower panel), show that Aβ addition significantly relieves the restriction on Fyn activation observed in untreated PrP-expressing controls relative to PrP-KO counterparts (cntr, grey and black bars, respectively). Conversely, no impact of Aβ was observed in PrP-KO neurons (see text for the explanation of the higher Fyn activation in untreated PrP-KO neurons). After seven days in culture, primary cortical neurons (isolated from PrP-expressing and PrP-KO mice lines Tg46 and F10, respectively [57] (Authorization n. 13/2012), and cultured as previously described [46]), were incubated (1 h, 37 °C), or not, with Aβ1–42 oligomers (5 µM, prepared and oligomerized as in [45]), and then lyzed using an ice-cold buffer containing glycerol (10% (w/v)), SDS (2% (w/v)), Tris/HCl (62.5 mM, pH 6.8), urea (1.8 M), Na3VO4 (5 mM), and cocktails of protease and phosphatase inhibitors (Roche). Total protein quantification, WB and densitometric analyses were accomplished as described elsewhere [45]. Here and after, values are expressed as mean ± SEM, and the number of replicates (i.e., different replicates from at least four primary cortical cultures prepared on different days) is indicated inside each bar diagram. * p < 0.05, ** p < 0.01 (Student’s t-test).