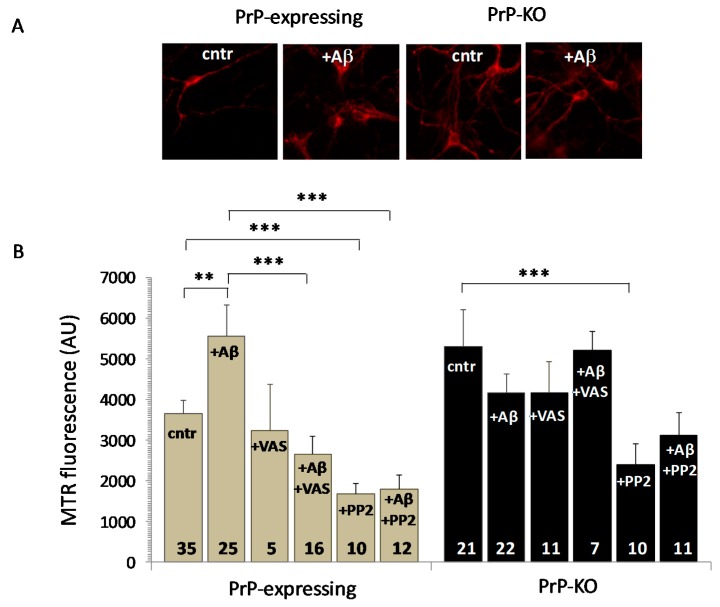
Figure 4.
Aβ1–42 causes a PrP-dependent mitochondrial ROS overload in PrP-expressing neurons through activation of NADPH oxidase. (A,B) Starting from the higher ROS quantity present in mitochondria of untreated PrP-KO neurons than in the PrP-expressing counterparts (cntr), Aβ addition (+ Aβ) increases mitochondrial ROS only in PrP-expressing neurons as clearly demonstrated by the representative fluorescence micrographs of neurons loaded with the mitochondrial ROS-sensitive dye MTR and acquired with a 40× objective (A), and the bar diagram reporting MTR fluorescence quantification (B). The source of such an Aβ-dependent supplementary ROS content in mitochondria of PrP-expressing neurons is the cytosolic NADPH oxidase, in light of ROS diminution upon inhibiting the enzyme with 3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo(4,5-d)pyrimidine (VAS 2870, 10 µM, 1 h, 37 °C) (+ Aβ + VAS). Also addition of the Fyn inhibitor 3-(4-chlorophenyl) 1-(1,1-dimethylethyl)-1H-pyrazolo(3,4-d)pyrimidin-4-amine (PP2, 10 µM, 1 h, 37 °C) reduces ROS presence in mitochondria of PrP-expressing neurons, either in the absence (+PP2) or the presence of Aβ oligomers (+ Aβ + PP2). This is consistent with the uphill stimulation of NADPH oxidase by Fyn and the relieve of the PrP-dependent block of Fyn activation by Aβ oligomers (Figure 1). However, the drastic ROS diminution observed in PrP-KO neurons treated with PP2 (+ PP2), but not with VAS (+ VAS), indicates that other Fyn-controlled (and PrP- and NADPH oxidase-independent) pro-oxidant pathways contribute to mitochondrial ROS accumulation. ** p < 0.01, *** p < 0.001 (Student′s t-test). Other details are as in the legend to Figure 1 and Figure 2.