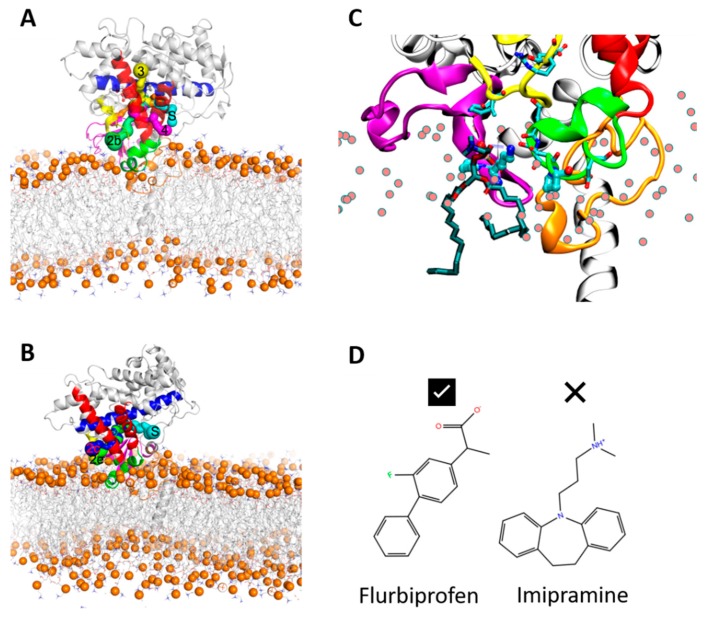
Figure 6.
Initial (A) and final (B) snapshots of the AA MD simulation (SIM1) of the apo form of CYP 2C9, showing tunnels accessible to a water molecule probe between the active site and the protein surface. Tunnel 2b (green) connects the active site and the membrane and is present in both snapshots, as is tunnel S (cyan). Tunnel 3 (yellow) is present in the initial snapshot and tunnel 2c (blue) is present in the final snapshot. The protein and bilayer are shown with the same color scheme as in Figure 5. (C) Close-up view of the entrance to the 2b tunnel showing how the phosphate group of a phospholipid molecule (shown in stick representation colored by atom type with cyan carbons) makes a hydrogen bond with the amino group of K72 (all other lipid molecules are represented by spheres for the phorphorous atoms only; the protein is represented and colored as in Figure 5). This interaction is important for pulling the phospholipid molecule somewhat out of the membrane towards the tunnel to the active site. This motion leads to partial opening of the β-sheet and the F’–G’ regions; further opening would be required for a substrate molecule to access the active site. (D) K72 may interact analogously with acidic substrates, such as flurbiprofen, a drug that is a substrate of CYP 2C9 (left), and may repel basic substrates such as the tricyclic antidepressant (TCA) drug, imipramine, which is a substrate of CYP 2C19 (right).