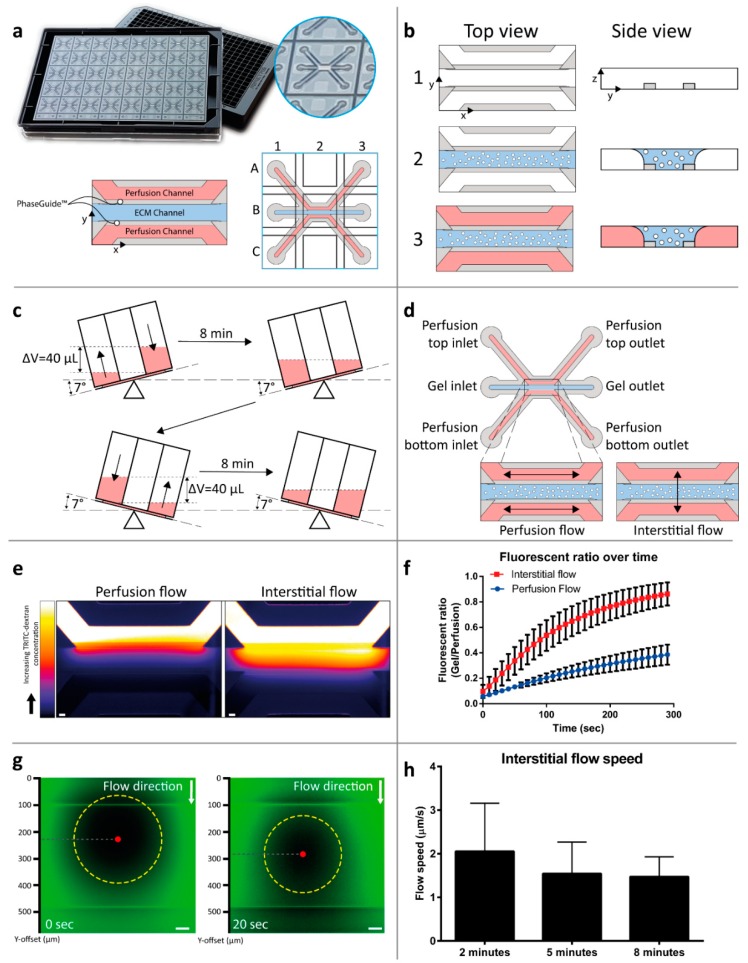
Figure 1.
Interstitial flow-on-a-chip characterization. (a) The microfluidic microtiter plate ‘OrganoPlate’ was used for a 3D cell culture, based on a 384 well plate interface with 40 microfluidic chips integrated in the bottom. A gel channel (blue) holds the extracellular matrix (ECM) in place through the PhaseGuide’s pressure barrier function. (b) Cells can be introduced in the middle lane (gel channel) to create a 3D cell culture with interstitial flow in a collagen ECM. Following the addition of a medium in the adjacent channels (2), the plate is placed on a tilted rocking platform with a rocking interval of 8 min, (c) which creates a height difference (equivalent to a 40 µL volume difference) resulting in gravity driven, continuous, and bi-directional perfusion of the cultures. (d) The resultant perfusion flow. (e) By placing the plate perpendicular to the perfusion flow condition, the flow is directed through the ECM gel in an interstitial flow. The interstitial flow was visualized by introducing a medium volume containing a fluorescent dye coupled to a dextran molecule in the top perfusion channel, thus, creating a pressure difference between the perfusion channels, image is acquired 2 min after introduction of the dye, scale bar = 100 µm). (f) Quantification was performed by calculation of the fluorescence ratio in the top perfusion and gel channels (plot represents average ± SD, n = 12–15 technical replicates). More detailed interstitial flow measurements within the ECM gel were performed using FRAP. (g) Images of the photobleached spot at two consecutive timepoints. The movement of the bleached spot (yellow circle) was tracked by the displacement of its center of mass (red dot). (h) The movement of the spot was calculated as an indication of the flow speed over time (n = 17 technical replicates, bar plots represent average + SD, scale bar = 50 µm).