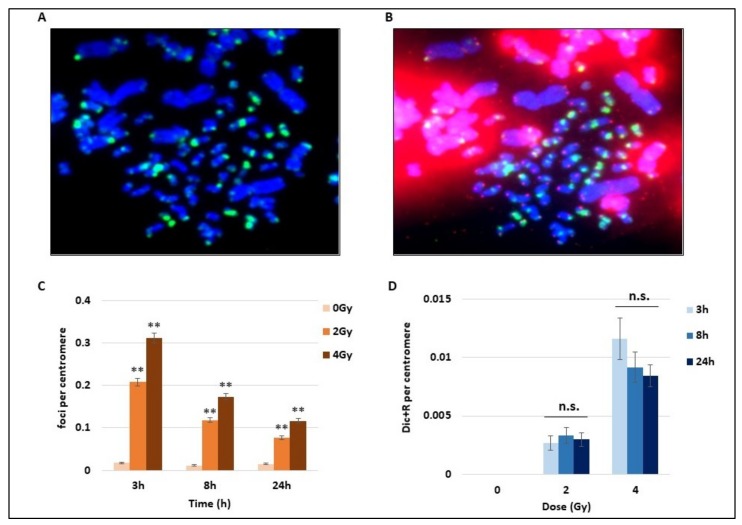
Figure 1.
Kinetics of DNA breaks signalling and unstable chromosome aberrations after radiation exposure. Blood samples from three donors were irradiated at 2 or 4 Gy or kept free from ionising radiation, analysed using the PCC technique and fixed 3 h, 8 h, or 24 h later. (A) Cells were first stained by immunofluorescence with a FITC (fluorescein isothiocyanate)-coupled γ-H2AX antibody (in green) used to detect foci on both human (1-chromatid chromosomes) and hamster chromosomes (2-chromatid chromosomes). (B) A second staining using PNA-FISH (Peptide Nucleic Acid Fluorescence In Situ Hybridisation) Telomere and Centromere probes was performed on the same Premature Chromosome Condensation (PCC) cells after the immunofluorescence to simplify the detection of chromosome unstable aberrations (Dicentric and Ring chromosomes and Acentric fragments). Centromeres are stained in green, while telomeres are in red, DNA appears in blue after Dapi staining. Dicentric chrosomes are chromosomes with two centromeres in green. (C) The number of γ-H2AX foci on human chromosomes was counted after immunofluorescence staining (cf image A) and expressed per centromere to exclude bias due to the possible loss of PCC fragments during the cytospin step. The increase of foci with 2 and 4 Gy irradiation (IR) is significant at 8 h and 24 h post-IR (** p < 0.01) and the bars represent standard error. The number of foci decreases with time. (D) Dicentric and ring chromosomes were counted after Telomere and Centromere FISH staining (as represented in B) and expressed per centromeres. The unstable chromosome aberrations increase with doses. There is no statistical difference between the different time points at 2 and 4 Gy (n.s).