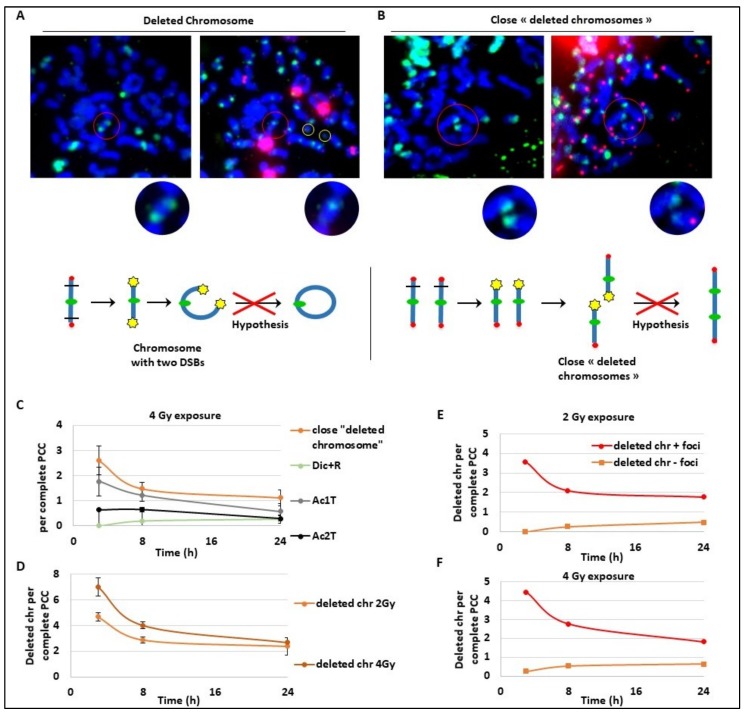
Figure 4.
Kinetics of late and slow DNA repair mechanisms. Blood samples from three donors were irradiated at 2 or 4 Gy or kept free from ionising radiation, analysed using the PCC technique and fixed 3 h, 8 h, or 24 h post-exposition. (A,B) Images of the same PCC exposed to 4Gy were observed after γ-H2AX immunofluorescence staining (in green on the left panels) and after TC staining (right panels). Centromeres are stained in green and telomeres in red after PNA-FISH staining. Red circles show the zones of interest and yellow circles indicate acentric fragments. A chromosome with two DSBs (A) and two chromosomes with close signalled DSBs (B) are both signalled by γ-H2AX and considered as “in repair”. In (A), the chromosome is expected to become a ring while in (B), the two chromosomes are expected to fuse and make a dicentric chromosome. Below each image, a schema represents the hypothetical mechanism of CA formation. On the schema, centromeres are represented with green circles, telomeres with red circles, and γ-H2AX foci with yellow stars. The red crosses on the hypothesis conclude that the data will not confirm the hypothesis. (C–F) Scoring was performed only on complete PCC cells containing between 45 and 48 fragments. (C) The frequency of “close deleted chromosomes”, Dic+R, Ac1T, and Ac2T, per PCC were reported at 3 h, 8 h, and 24 h after 4 Gy irradiation. (D) The frequency of deleted chromosomes (with one telomere only) was followed after 2 and 4 Gy irradiation at three time points: 3 h, 8 h, and 24 h post-IR. (E,F). The proportion of deleted chromosomes containing γ-H2AX signalling or not signalled was quantified after 2 Gy (E) and 4 Gy (F) exposure.