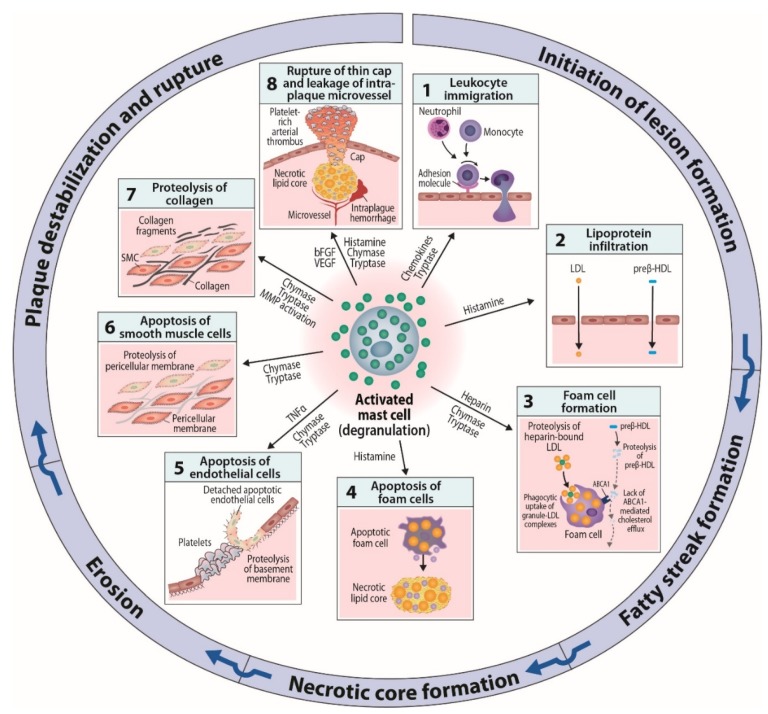
Figure 2.
Potential effects of intimal mast cells on initiation and progression of human atherosclerotic lesions. I. Initiation of lesion formation. (1). Activated subendothelial mast cells contribute to leukocyte recruitment by releasing chemokines, and by also releasing tryptase and TNFα which enhance adhesion molecule expression on endothelial cells. (2). Activated mast cells release vasoactive substances, notably histamine, which increase endothelial permeability for low-density lipoprotein (LDL) and high-density lipoprotein (HDL) particles. II. Fatty streak formation. (3). Subendothelially, LDL particles bind to the heparin component of exocytosed mast cell granules, after which granule chymase proteolyzes the particles and renders them unstable and susceptible to fuse with each other. When macrophages phagocytose such complexes composed of granule-bound fused LDL particles, they become filled with LDL-derived cholesterol and are converted to foam cells filled with cholesteryl ester-containing lipid droplets. The granule neutral proteases chymase and tryptase degrade preβ-HDL particles, which thereby lose their ability to interact with the ABCA1 transporter on macrophage foam cells and to accept cholesterol from the foam cells. III. Necrotic core formation. (4). Mast cell-derived histamine is able to induce macrophage apoptosis. When a macrophage foam cell dies, the generated cellular debris and the liberated lipid droplets contribute to the formation of an extracellular necrotic lipid core. The formation of a core is the hallmark of conversion of an early fatty streak lesion into an advanced atherosclerotic plaque, which consists of a core and a collagen cap. The cap separates the strongly thrombogenic core from the circulating blood. IV Erosion. (5). Release of tryptase and/or chymase by activated subendothelial mast cells induce degradation of endothelial basement membrane with ensuing apoptosis and detachment of the involved endothelial cells. Mast cell-derived TNFα contributes to endothelial apoptosis, while mast cell-derived heparin tends to attenuate the growth of the forming platelet-rich arterial thrombus at the site of erosion. V. Plaque destabilization and rupture. (6). Activated mast cells in the collagen cap release chymase, which degrades the pericellular matrix of smooth muscle cells with ensuing apoptotic death of the cells due to loss of outside-in survival signaling. Loss of the collagen-producing smooth muscle cells reduces net collagen formation in the cap, and so weakens it. (7). Release chymase and tryptase by mast cells in the collagen cap locally activates extracellularly located proforms of matrix metalloproteinases, and so triggers collagen degradation which further weakens the cap and destabilizes the plaque. (8). Mast cell-derived angiogenic factors induce growth of microvessels into the hypoxic regions of the otherwise avascular plaque. Mast cell-derived tryptase and chymase, again, may degrade the fragile microvessel walls, and so trigger microvascular hemorrhage which contributes to plaque instability. Together, cap thinning and intraplaque hemorrhages render the plaque susceptible to rupture with ensuing formation of a large lumen-occluding coronary thrombus.