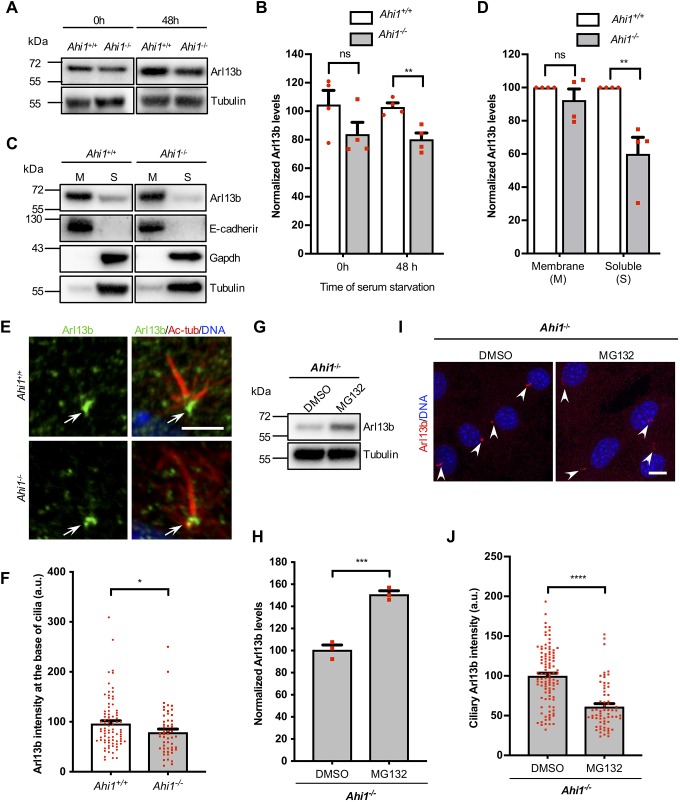
Fig. 5.
Loss of Ahi1 decreases Arl13b levels in soluble cell fractions and at the ciliary base. (A) Immunoblotting of MEF lysates from Ahi1+/+ and Ahi1−/− cultures grown in 10% FBS (0 h) and serum-starved for 48 h probed for Arl13b and tubulin. (B) Quantification of chemiluminescent signals normalized to Ahi1+/+ values; tubulin was used as a loading control. n=4/genotype and experiments were performed in duplicate. (C) Arl13b analysis in membrane (M) and soluble (S) fractions by immunoblotting in Ahi1+/+ and Ahi1−/− cell cultures. E-cadherin was used as a control for membrane-bound proteins and Gapdh as a control for soluble proteins. (D) Quantification of Arl13b signals in n=4 cell lines/genotype, experiments were performed in duplicate. Tubulin was used as a loading control for M and S fractions, and bars represent normalization to Ahi1+/+ values. (E) Ahi1+/+ and Ahi1−/− MEFs immunolabeled for Arl13b (green) and acetylated α-tubulin (Ac-tub; red) with DNA/nuclei (blue). Cells were pre-extracted with buffer containing 0.5% Triton X-100 and fixed with methanol. Scale bar: 3 µm. Arrows highlight Arl13b immunolabeling at the ciliary base. (F) Arl13b intensities at the ciliary base normalized to Ahi1+/+ values. (G,I) Immunoblotting and immunofluorescence of Arl13b in Ahi1−/− MEFs treated with vehicle (DMSO) or MG132 for 6 h. Prior to MG132 treatment, Ahi1−/− MEFs were serum-starved (48 h) to induce formation of primary cilia (I). Arl13b, red, arrowheads, and DNA/nuclei, blue. Scale bar: 10 µm. (H,J) Quantitative analysis of Arl13b chemiluminescence in cells (H) and Arl13b fluorescent signals at primary cilia (J). Experiments were carried out in n=3 cell lines in duplicate. Bars represent values normalized to controls (DMSO/vehicle) and error bars represent s.e.m. ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; ns, not significant [Mann–Whitney test in B (0 h), D (M), F and J, and unpaired two-tailed t-tests B (48 h), D (S), and H].