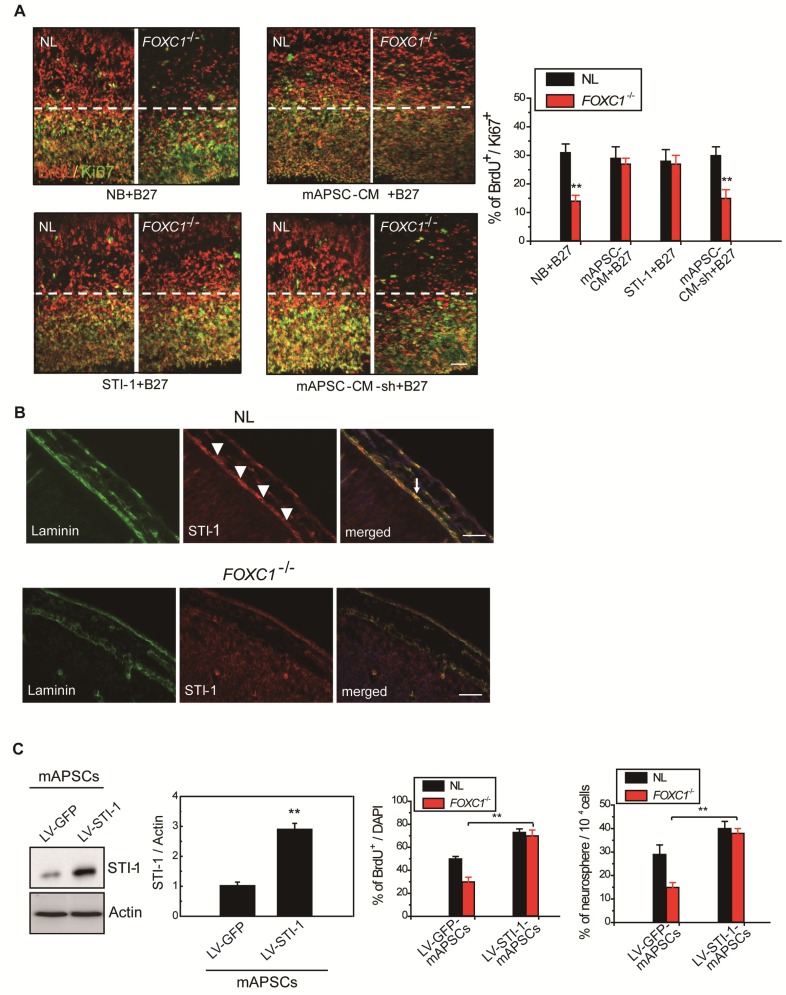
Figure 5.
Meningeal-derived STI-1 rescues deficient cortical neurogenesis caused by lack of FOXC1. (A) Representative images of BrdU+/Ki67+ double immunostaining; the dotted line demarcates the transition from proliferative and postmitotic zones in the ventricular wall. In co-culture experiments, a significantly enhanced proportion of BrdU+/Ki67+ cells were found in FOXC1-/- forebrain explants embedded in mAPSCs-CM, but not in that of NLs. The cortex of FOXC1-/-brain explants treated with STI-1 (100 ng/mL) exhibited increased cell cycle exit; no increase was observed in brain explants of NLs. However, rescue of the cell-cycle exit phenotype in the FOXC1-/- brain explant was inhibited in the NL-mAPSC-CM-sh. (B) In an immunohistochemistry study (E14.5), a higher STI-1 expression that colocalized with laminin was observed in the meninges of NLs compared to FOXC1-/- mice meninges. STI-1 signal was co-expressed with LacZ staining in the FOXC1+/- heterozygous mice meninges. (C) Significant overexpression of STI-1 was found in LV-STI-1 transduction in NL-mAPSCs (LV-STI-1-NL-mAPSCs) compared with the control (LV-GFP-NL-mAPSCs). Reversion of the FOXC1-/- SVZ neurosphere phenotype after co-culture with LV-STI-1-NL-mAPSCs was demonstrated by increased numbers of neurosphere formation and percentage of BrdU+ cells. Data are expressed as the mean ± SEM. * P < 0.05 and ** P < 0.01 vs. control, Bar = 40 μm