Figure 1.
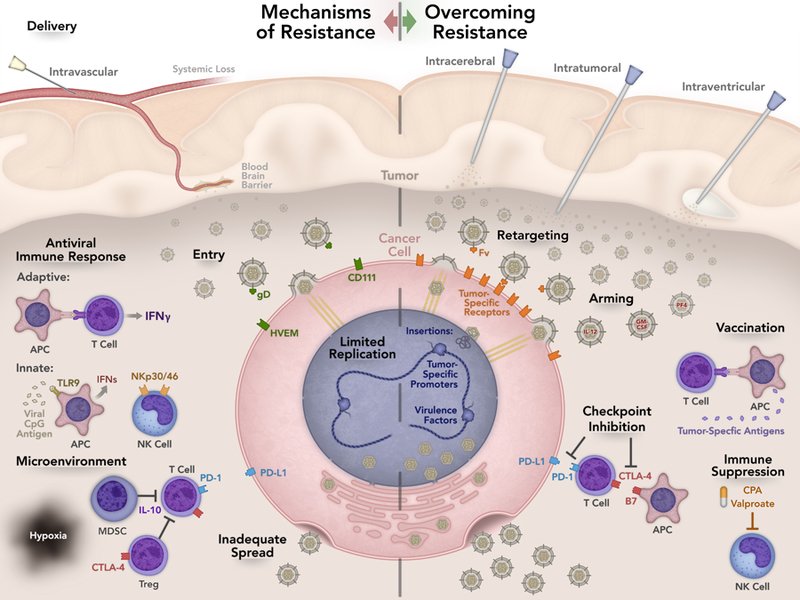
Summary of mechanisms of resistance and strategies to overcome resistance. Suboptimal delivery is one possible mechanism of treatment failure. Intratumoral injection is the most common route of delivery but other routes have been tested. Intravascular and intraventricular delivery are currently being investigated and may allow access to otherwise inaccessible tumors. Efficient entry is imperative for oHSV action. CD111 is the most efficient entry receptor but expression varies among tumors. To enhance targeting of oHSV, viral retargeting techniques to tumor specific antigens are being investigated. Viral replication and spread is necessary to prolong viral infection and effectively maximize the anti-tumor response. Host recognition of the virus and degradation within vesicles prevents viral infection. Chimeric HSVs, HDAC6 inhibition, and STAT3 expression can alter oHSV replication. The tumor microenvironment contains immune and endothelial cells, fibroblasts, pericyte cells, and neurons, astrocytes, and microglia. The extracellular matrix created by these cells aids in tumor growth, progression, invasion, and metastasis. Altering the tumor microenvironment can help modulate the anti-tumor response. The host anti-viral immune response may decrease viral efficacy and development of an anti-tumor response. Antigen presenting cells (APCs) recognize viral antigen, which leads to interferon (IFN) production resulting in less viral replication. Suppressing the innate immune response to the virus decreases IFN production resulting in greater viral efficacy. Vaccination with tumor antigens can augment the immune response to the tumor. Arming oHSVs with various cytokines in addition to checkpoint inhibition allows sustained T cell activation contributing to a robust anti-tumor response.
