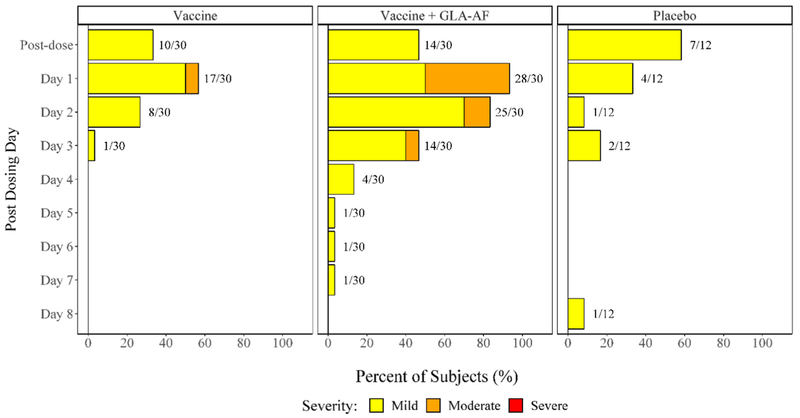
Fig. 4.
Percent of Subjects Experiencing Solicited Injection Site Symptoms by Treatment Group, Severity, and Day Post-Vaccination, Post Any Dose. NOTE: A linear mixed effects model estimated the mean duration of injection site symptoms for a person receiving vaccine with GLA-AF to be 1.33 days longer than that of one receiving placebo (p < 0.001) and 1.22 days longer than one who received vaccine alone (p < 0.001).