Abstract
Walker Warburg syndrome (WWS) lies at the severe end of the spectrum of the congenital muscular dystrophies. WWS is a congenital disorder of the O-glycosylation that disrupts in the post-translation modification of dystroglycan proteins. WWS is characterized by the involvement of the central nervous system and rarely by multisystem involvement. Next-generation sequencing discovered that multiple genes are associated with this disorder. FKTN is the rarest cause of WWS. We describe a clinical-autopsy report of a molecularly- confirmed WWS case presenting with ventriculomegaly, agenesis of the corpus callosum with a novel phenotype of Dandy-Walker malformation and unilateral multi-cystic kidney. The whole-exome sequencing confirmed a homozygous variant (c.411C>A) in the FKTN gene with a premature termination codon. This case emphasizes the importance of detailed postnatal phenotyping through an autopsy in any pregnancy with antenatally identified malformations. Obstetricians, pediatricians as well as fetal medicine experts need to counsel the parents and focus on preserving the appropriate sample for genetic testing. WWS, though rare deserves testing especially in the presence of positive family history. Dandy-Walker malformation is a novel feature and expands the phenotypic spectrum.
Keywords: Congenital Disorder of Glycosylation, Walker-Warburg Syndrome, Hydrocephalus
CASE REPORT
A non-consanguineously married couple presented to the genetic center with fetal malformations detected on the level II ultrasound. The nuchal translucency measured at 12 weeks was 1.1 mm (Reference value [RV] less than 2.5 mm) at a crown-rump length (CRL) of 66 mm. The anomalies detected on the level II scan at 21 weeks of gestation included bilateral ventriculomegaly (right ventricle -18.1 mm; left ventricle - 20.1 mm (RV<10 mm) and dangling choroids. The posterior fossa showed a direct communication between the fourth ventricle and the cisterna magna, suggestive of Dandy-Walker malformation (DWM). The right kidney was multi-cystic and measured 45 mm (RRange; 16-30). The left kidney had a normal shape and size. Right-sided congenital talipes equinovarus was also identified. Their first pregnancy was terminated due to an antenatally detected ventriculomegaly at 18 weeks. Unfortunately, a genetic study was not performed in that opportunity.
Due to the current pregnancy ultrasonographic findings, the couple was advised to perform genetic testing to identify the likely etiology and were informed on the dismal prognosis of the fetus. The pregnancy was terminated with 26 weeks of gestation, and the couple authorized a post-natal evaluation and autopsy.
Autopsy Findings
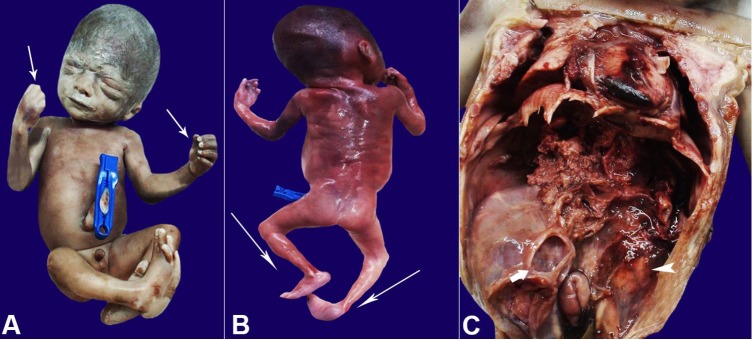
On the corpse gross examination, there was no facial dysmorphism (Figure 1A). Fetal weight was 652 gm, the head circumference was 26 cm CRL 23.5 cm, all these measurements corresponded to 25-26 weeks of gestation. Contractures were noted in the fingers and bilateral elbow joints (Figure 1A, 1B). Right-sided congenital talipes equinovarus was present, along with a rocker bottom foot on the left side (Figure 1B).
Figure 1. A – Gross view (front profile) of the fetus showing fetal face. Note the contractures in the fingers (white arrows) as well as the elbows; B – Gross view (back profile) of the fetus showing right-sided congenital talipes equinovarus, along with a rocker bottom foot on the left side (white arrows); C – Gross view of the opened abdominal and thoracic cavities showing an enlarged multicystic right kidney (white arrow) and a normal size left kidney (arrowhead).
The abdomen was opened with a paramedian incision. The liver, spleen, and intestine were grossly normal. On dissection in the retroperitoneal fossa, the right kidney was grossly enlarged, occupying almost half of the abdominal cavity (Figure 1C). The left kidney was normal in appearance and on the cut section. Both ureters and bladder were normal. No genital anomalies were identified.
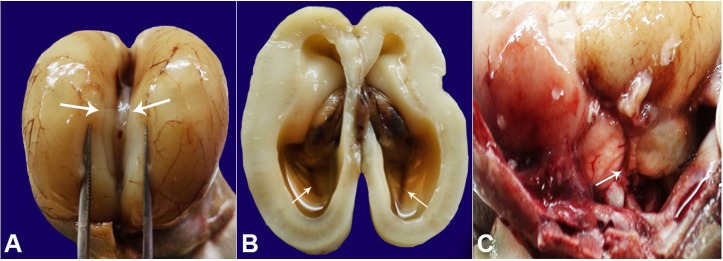
The cranial cavity was opened and examined. Grossly the cerebral hemispheres were normal. However, the agenesis of the posterior part of the corpus callosum was present, better appreciated on separating the two cerebral hemispheres (Figure 2A). At an axial section of the brain, a severe bilateral ventricular dilatation was depicted (Figure 2B). The incision was extended posteriorly up-till the cerebellum and cervical part of the spinal cord. The posterior fossa revealed enlarged cisterna magna and a DWM (Figure 2C). Cardiac dissection did not reveal abnormalities, and the remaining systemic examination was normal.
Figure 2. Gross examination of the brain showing in A – the forceps separating the two cerebral hemispheres with agenesis of corpus callosum (white arrows); B – Axial section of the brain showing severe bilateral ventriculomegaly (white arrows); C – Posterior fossa of the brain vermian hypoplasia.
A chromosomal microarray (AGILENT -180K) was reported to be normal. Due to the recurrence of fetal malformation in subsequent pregnancies, a strong suspicion of a genetic disorder was raised, and therefore a trio whole-exome sequencing was advised.
Molecular Testing
Trio whole-exome sequencing revealed a homozygous variant, c.411C>A; (p.Cys137Ter) (ENST00000223528.2) (chr9:108366537C>A) in the FKTN gene. The p.Cys137Ter variant has a minor allele frequency of 0.02%, 0.004% in the 1000 genomes, ExAC. The reference codon is conserved across mammals. It is likely pathogenic according to the modified ACMG criteria (PVS1+PS4+PP3+PP4+PP5), known to cause congenital muscular dystrophy-dystroglycanopathy with brain and eye anomalies (type A).
The variant was validated by Sanger sequencing, and the segregation analysis in the parents revealed the variant to be present in a heterozygous state in both the mother and the father.
DISCUSSION
Impaired O-glycosylation of the alpha-dystroglycan causing congenital muscular dystrophies is a clinically and genetically heterogeneous group of autosomal recessive muscular dystrophies with variable neurological and ophthalmic involvement.1
Fukuyama congenital muscular dystrophy represents a continuum of phenotypes, starting from the mildest Fukuyama muscular dystrophy, the more severe muscle-eye-brain disease to the antenatally detectable most severe Walker-Warburg-syndrome (WWS).2 Specific clinical criteria have been described to define the WWS.3 We present our patient as the WWS due to the severe fetal phenotype.
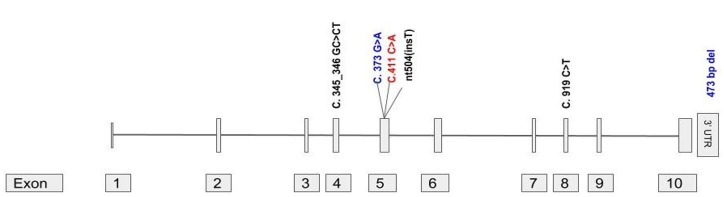
To the best of our knowledge, only 5 families with FKTN gene associated Walker-Warburg syndrome have been reported so far4-7 (Figure 3). We report the first clinic-autopsy-molecular correlation of the WWS caused by the FKTN gene. DWM was identified in the fetus, which had not been earlier described, thus expanding the phenotypic spectrum of the disease. The most striking finding was the presence of an enlarged multicystic dysplastic kidney that was also identified in our case. The renal involvement, observed in our case, can be explained due to defective glycosylation of the dystroglycans, also occurring in the renal parenchyma, as the FKTN gene is also expressed in the kidney. Antenatal presentation of cystic kidneys has been earlier described in the literature in association with glycosylation defects.8-10 The uniqueness of the present case also lies in unilateral renal involvement. This is rather unusual for a genetic disorder which generally presents with symmetrical organ involvement. One explanation could be the co-existence of a unilateral pelvic-ureteric junction obstruction and causing a multicystic dysplastic kidney (MCDK). Ureteropelvic junction obstruction also one of the commonest abnormalities detected by antenatal ultrasound with an incidence of approximately 1 in 3640 births.11 Another possibility is the occurrence of somatic renal epithelial lining leading to cystic kidneys or a local epigenetic mechanism causing the disease.
Figure 3. Gene structure of FKTN (Fukutin) gene (NM_006731, Exons 10) with previously reported exonic mutations for WWS. Variants in Black are homozygous in nature, blue represents compound heterozygous variants, and exonic homozygous mutation identified in this case is coloured Red.
The variant in the FKTN gene, c.411C>A detected on WES leads to premature truncation of the protein at the 137th amino acid. This variant has been reported previously by Sframeli et al.12 The mutational spectrum of the FKTN gene has been shown in Figure 2. Interestingly half of these are present in exon 5. Further, molecularly diagnosed cases are required to establish this as a hot spot.
Genetic Counseling
This disorder follows an autosomal recessive mode of inheritance. The risk of recurrence for the disorder in the family is 25% in subsequent pregnancies. Options of prenatal, as well as pre-implantation genetic diagnosis, should be explained to the parents.
CONCLUSION
In conclusion, this report adds to the phenotypic spectrum of Walker-Warburg syndrome. Further, the truncating variant causing a severe phenotype establishes the genotypic-phenotypic correlation. We also aim to highlight the importance of saving the crucial samples after a detailed post-evaluation for genetic testing, especially in the presence of positive significant family history to future reproductive counseling.
Footnotes
How to cite: Arora V, Bijarnia-Mahay S, Kulshreshtra S, Singh K, Puri RD, Verma IC. Prenatal presentation of a rare genetic disorder: a clinical, autopsy and molecular correlation. Autops Case Rep [Internet]. 2019 Oct-Dec;9(4):e2019124. https://doi.org/10.4322/acr.2019.124
The authors retain an Informed consent signed by the parents.
Financial support: None
REFERENCES
- 1.Muntoni F, Brockington M, Blake DJ, Torelli S, Brown SC. Defective glycosylation in muscular dystrophy. Lancet. 2002;360(9343):1419-21. 10.1016/S0140-6736(02)11397-3. [DOI] [PubMed] [Google Scholar]
- 2.Falsaperla R, Praticò AD, Ruggieri M, et al. . Congenital muscular dystrophy: from muscle to brain. Ital J Pediatr. 2016;42(1):78. 10.1186/s13052-016-0289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobyns WB, Pagon RA, Armstrong D, et al. . Diagnostic criteria for Walker-Warburg syndrome. Am J Med Genet. 1989;32(2):195-210. 10.1002/ajmg.1320320213. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey C, Clement E, Mein R, et al. . Refining genotype phenotype correlations in muscular dystrophies with defectiveglycosylation of dystroglycan. Brain. 2007;130(Pt 10):2725-35. 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- 5.Cotarelo RP, Valero MC, Prados B, et al. . Two new patients bearing mutations in the fukutingene confirm the relevance of this gene in Walker-Warburg syndrome. Clin Genet. 2008;73(2):139-45. 10.1111/j.1399-0004.2007.00936.x. [DOI] [PubMed] [Google Scholar]
- 6.Silan F, Yoshioka M, Kobayashi K, et al. . A new mutation of the fukutin gene in anon-Japanese patient. Ann Neurol. 2003;53(3):392-6. 10.1002/ana.10491. [DOI] [PubMed] [Google Scholar]
- 7.Bernabé DB, Van Bokhoven H, Van Beusekom E, et al. . A homozygousnonsense mutation in the fukutin gene causes a Walker-Warburg syndrome phenotype. J Med Genet. 2003;40(11):845-8. 10.1136/jmg.40.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabhan MM, ElKhateeb N, Braun DA, et al. . Cystic kidneys in fetal Walker-Warburg syndrome with POMT2 mutation: intrafamilial phenotypic variability in four siblings and review of literature. Am J Med Genet A. 2017;173(10):2697-702. 10.1002/ajmg.a.38393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora V, Puri RD, Bhai P, et al. . The first case of antenatal presentation in COG8-congenitaldisorder of glycosylation with a novel splice site mutation and an extendedphenotype. Am J Med Genet A. 2019;179(3):480-5. 10.1002/ajmg.a.61030. [DOI] [PubMed] [Google Scholar]
- 10.Arora V, Shah N, Khatter S, et al. . ALG9 Associated Gillessen-Kaesbach-Nishimura Syndrome (GIKANIS): an uncommon aetiology of enlarged foetal kidneys. J Foetal Med. 2018;5(4):237-9. 10.1007/s40556-018-0183-1. [DOI] [Google Scholar]
- 11.Aslam M, Watson AR. Unilateral multicystic dysplastic kidney: long term outcomes. Arch Dis Child. 2006;91(10):820-3. 10.1136/adc.2006.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sframeli M, Sarkozy A, Bertoli M, et al. . Congenital muscular dystrophies in the UK population: clinical and molecular spectrum of a large cohort diagnosed over a 12-year period. Neuromuscul Disord. 2017;27(9):793-803. 10.1016/j.nmd.2017.06.008. [DOI] [PubMed] [Google Scholar]