Abstract
Chordoma is a rare tumor. It has unique clinical, pathological and immunohistochemical characteristics. Accurate diagnosis is essential as the tumor shows an aggressive clinical course and requires a multimodal therapeutic approach. A case with wide spread distant metastatic disease that was initially thought to represent metastatic thyroid carcinoma is presented. Appropriate clincopathologic correlation and the histologic findings raised the possibility of poorly differentiated chordoma. The diagnosis was confirmed by immunohistochemistry for INI-1 and Brachyury. The approach to the diagnosis emphasizing the clinical and pathologic findings of this case is discussed and reviewed in the context of the published literature.
Keywords: Chordoma, Notochord, SMARCB1 Protein
INTRODUCTION
Chordoma is an aggressive tumor originating from embryonal notochordal remnants. In adults, it most commonly involves the sacrococcygeal area whereas in children it affects mostly the skull base.1 It shows a slight male predominance with a peak incidence between 50-60 years of age.2 It is quite rare in children and adolescents.3 Although not yet included as a distinct entity in the current World Health Organization Classification of Soft Tissue and Bone Tumors, poorly differentiated chordoma is a newly recognized subtype of chordoma that is more common in the pediatrics age group and shows more aggressive biologic behavior compared to other types of chordoma.4-6 A characteristic feature is the loss of SMARCB1-INI1 expression by immunohistochemistry. Although this immunohistochemical staining finding was previously described in a minority of chordoma cases, the clinical features associated with those cases may suggest that they probably belong to the poorly differentiated chordoma category.6,7 Herein, we present a case of poorly differentiated chordoma arising in the cervical spine in a thirty-year-old man who was recently diagnosed with papillary thyroid carcinoma. We report the occurrence of this tumor in association with other malignancies and we discuss the diagnostic approach, the pathologic findings and the differential diagnosis.
CASE REPORT
A thirty-year-old man with a history of papillary thyroid carcinoma with cervical lymph nodes metastasis, who underwent total thyroidectomy with lymph nodes dissection few months earlier, presented with progressive left shoulder and arm pain associated with paresthesia. No other neurological symptoms were present. On physical examination, a few enlarged cervical lymph nodes were present. Neurological physical examination was normal with intact sensory and motor functions in both upper and lower limbs. Laboratory tests were normal. Serum thyroglobulin level was low. CT scan and MRI studies showed a left paraspinal mass, which was centered in the left side of C6 vertebral body with extension from C4 to C7. It was associated with a large extra-osseous soft tissue component. Multiple enlarged mediastinal lymph nodes, lung nodules and other lytic bone lesions were seen. The initial clinical diagnosis was compatible with widespread metastasis from the papillary thyroid carcinoma versus primary malignant nerve sheath tumor originating from the left cervical paraspinal area. An Endoscopic ultrasound guided fine needle aspiration of the paratracheal and subcarinal lymph nodes showed scattered granulomas with no sign of malignancy. An excisional biopsy from a lung nodule was subsequently performed.
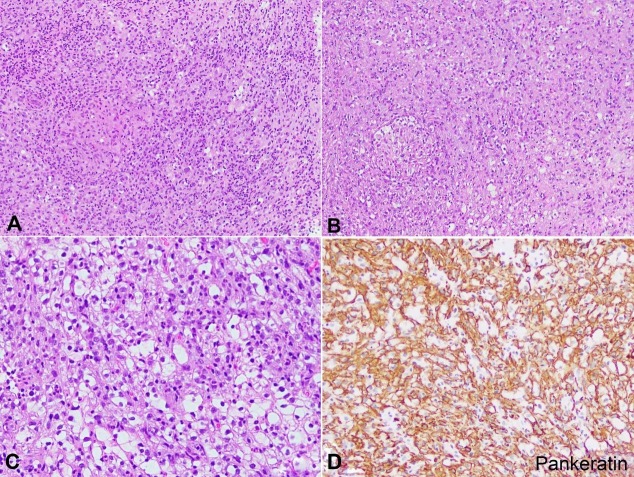
Histologic examination of the lung nodule showed a tumor composed of sheets of epithelioid to spindle cells exhibiting moderate amount of eosinophilic, pale cytoplasm with irregular and vesicular nuclei (Figure 1A). Scattered non-necrotizing, well-formed granulomas were present (Figure 1B). Prominent tumor necrosis was seen. Of note, a subset of tumor cells showed abundant cleared cytoplasm with smaller nuclei (Figure 1C). An initial panel of immunohistochemical stains revealed the tumor cells to be strongly and diffusely positive for pankeratin, cytokeratin19 (CK19) and epithelial membrane antigen (EMA) (Figure 1D). The tumor cells were negative for thyroid transcription factor-1 (TTF-1), Paired box-8 (PAX8) and thyroglobulin. The diagnosis was initially rendered as metastatic poorly differentiated carcinoma, and given the patients’ known history of thyroid carcinoma; a primary thyroid origin was favored despite the negative immunohistochemistry and the low level of serum thyroglobulin.
Figure 1. Photomicrographs of the lung nodule. A – Poorly differentiated chordoma. The tumor is composed of sheets of epithelioid cells with eosinophilic cytoplasm (H&E, 100X); B – Poorly-differentiated chordoma. Notice the well-formed granuloma and the cells with clear cytoplasm in the lower right corner (H&E, 100X); C – Poorly-differentiated chordoma. Focal areas show tumor cells with clear to vacuolated cytoplasm admixed with epithelioid cells (H&E 200X); D – Pankeratin immunohistochemical stain highlighting the lesional cells (Pankeratin immunohistochemical stain, 100X).
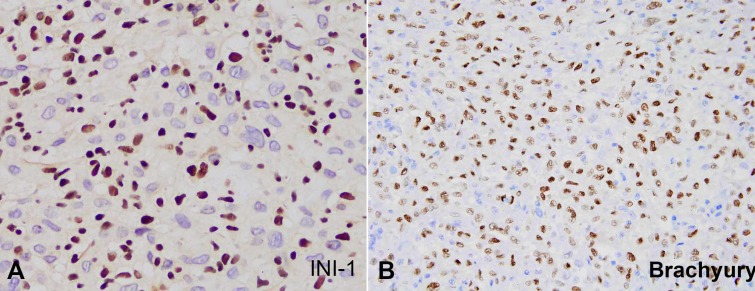
The patient subsequently developed new bone lesions and a new left cervical mass. Biopsy from the cervical mass showed essentially similar histomorphologic and immunohistochemical features. The well-formed non-necrotizing granulomas were also present. However, an extended panel of immunohistochemical stains was performed and showed negative staining for P63, cytokeratin 5/6 (CK5/6), S100 and CD34. INI-1 showed loss of expression in the tumor cells while it was intact in the inflammatory cells (Figure 2A). At this point, the differential diagnosis was expanded to include tumors that show keratin expression with loss of INI-1. The paraspinal mass was favored to represent a primary tumor rather than a metastatic process. The differential diagnosis included epithelioid malignant peripheral nerve sheath tumor, metastatic epithelioid sarcoma and poorly-differentiated chordoma. Malignant extrarenal rhabdoid tumor was less likely given the age of the patient. No soft tissue masses were present in the upper or lower limbs by imaging studies to suggest metastasis form a soft tissue sarcoma. The histologic features along with the negative S100 and CD34 staining ruled out the diagnosis of epithelioid malignant peripheral nerve sheath tumor. Given the age of the patient, the location of the mass, the histologic features of a poorly-differentiated tumor with scattered clear to vacuolated cells and the positive staining for keratin, EMA and loss of INI-1; poorly differentiated chordoma originating from the cervical spine and metastasizing to the lung, bone and cervical lymph nodes was strongly favored. Immunohistochemical stain for brachyury, performed at an outside facility, was positive confirming the diagnosis (Figure 2B).
Figure 2. Photomicrographs of the lung nodule. A – INI-1 immunohistochemical stain in poorly differentiated chordoma. Notice the loss of expression in the tumor cells and the intact expression in the inflammatory cells (400X); B – Brachyury immunohistochemical stain in poorly differentiated chordoma (200X).
DISCUSSION
According to the current World Health Organization Classification of Soft Tissue and Bone Tumors, chordoma is classified into three subtypes: conventional, chondroid and dedifferentiated.8 The conventional subtype is characterized by the presence of the physaliferous cells that are embedded in abundant extracellular myxoid matrix. The chondroid subtype refers to tumors with a matrix that mimics hyaline cartilaginous tumors. The dedifferentiated chordomas shows a biphasic morphology; typical features of chordoma with an abrupt juxtaposition to a high-grade undifferentiated spindle cell tumor or osteosarcoma.8
Poorly differentiated chordoma is a newly recognized subtype of chordoma.4,5 It is an exceptional neoplasm with very rare case series described.2,4 These tumors are not yet included as a distinct tumor entity in the World Health Organization classification of soft tissue and bone tumors. It is more aggressive and quicker developing than the conventional chordoma. This tumor has an increased tendency to occur in the skull base and cervical spine.2 Acknowledgment of this subtype is vital because these malignancies should be managed aggressively with multimodality treatment. Compared to other chordoma subtypes, poorly differentiated chordoma has a significantly decreased mean overall survival and is characterized by a different clinical and immunohistochemical phenotype; with a distinctive SMARCB1/INI1 nuclear expression loss.6 Poorly-differentiated chordoma arises in children and adolescents much more commonly than older patients, mostly affecting children under five years of age, with a slight female predominance.1,2
The differential diagnosis of poorly-differentiated chordoma may be challenging. This includes metastatic carcinoma, epithelioid sarcoma, epithelioid malignant peripheral nerve sheath tumor, and in children, myoepithelial carcinoma and extrarenal malignant rhabdoid tumor. Most of these tumors essentially show close histomorphologic features that pose diagnostic challenges based on the hematoxylin and eosin stain alone. However, attention should always be made to the clinical setting and the radiologic findings in each case (Table 1). Immunohistochemical stains are invaluable for the diagnosis. Although many of these tumors show overlapping staining patterns (Table 2), brachyury is believed to be a sensitive and specific marker for notochordal origin.2
Table 1. Clinical and pathologic features of the tumors included in the differential diagnosis for poorly differentiated chordoma.
| Tumor | Clinical setting | Pathology |
|---|---|---|
| Carcinoma | Older patients. A primary site is often detected by imaging studies. | Variable morphology depending on the type of carcinoma and site of origin. Immunohistochemical stains often help in determining the site of origin. |
| Conventional type epithelioid sarcoma | Involves the distal extremities of young adults (median age: 30 years), involves the skin, subcutis, tendons or fascia.9 | Epithelioid to occasionally spindle cell proliferation with vesicular nuclei and small nucleoli, pseudogranulomatous appearance |
| Proximal type epithelioid sarcoma | Arises in the deep soft tissues of the pelvis and perineum. Older patients compared to conventional epithelioid sarcoma. | Infiltrative, multinodular sheets of epithelioid cells with prominent atypia, rhabdoid morphology and prominent nucleoli. Pseudogranulomatous appearance is not seen. |
| Epithelioid malignant peripheral nerve sheath tumor | Most often arises in the subcutaneous tissue of young to middle aged adults. Mostly in the extremities. May arise in association with a large nerve. | Well-circumscribed, multinodular growth pattern. Relatively uniform cells with abundant eosinophilic cytoplasm, vesicular nuclei and single prominent nucleolus. The stroma might be myxoid. |
| Extrarenal malignant rhabdoid tumor | Affects infants and children. Rapidly enlarging mass that involves the deep soft tissues in axial locations (neck, paraspinal areas, perineum abdominal and pelvic cavities).8 | Sheets of tumor cells with rhabdoid morphology with large vesicular nuclei with prominent nucleoli. Juxtanuclear eosinophilic, PAS positive-diastase resistant hyaline globules are seen.8 |
| Myoepithelial carcinoma | Predominates in the pediatric population. Mostly in the extremities.10 | The tumor shows heterogeneous morphology with epithelioid (usually the predominant pattern), clear, spindle and/or plasmacytoid cells forming nests, cords or solid sheets in a myxoid or hyalinized stroma. |
Table 2. Immunohistochemical stains for the differential diagnosis of poorly differentiated chordoma.
| Tumor | Keratin | S100 | INI-1 | Brachyury |
|---|---|---|---|---|
| Carcinoma | + | - | Intact* | - |
| Epithelioid sarcoma | + | - | Lost | - |
| Epithelioid malignant peripheral nerve sheath tumor | + | + | Lost in 50%.11 | - |
| Extrarenal malignant rhabdoid tumor | + | +/- | Lost | - |
| Myoepithelial carcinoma | + | +/- | Lost in 10-40%.10,11 | - |
| Poorly differentiated chordoma | + | +/- | Lost | + |
Clinically, poorly differentiated chordoma are considered more aggressive than conventional chordoma. PDC tends to have rapid onset of local recurrence and metastasis. Although conventional chordoma may present with distant metastasis, this is believed to occur late in the course of disease due to the indolent and slow growing nature of conventional chordoma. According to one study,2 when compared to spinal conventional chordoma and chondroid chordoma, spinal poorly differentiated chordoma shows significantly decreased mean overall survival, decreased mean progression free survival, decreased mean local control time and decreased mean metastasis free survival (Table 3). Therefore, poorly differentiated chordoma should be treated aggressively with a multimodality therapy.
Table 3. Prognostic parameters of spinal poorly differentiated chordoma compared to spinal conventional and chondroid chordoma2.
| Spinal poorly differentiated chordoma | Spinal conventional chordoma | Spinal chondroid chordoma | P value* | |
|---|---|---|---|---|
| Mean overall survival | 46 months | 129 months | 101 months | 0.035 |
| Mean progression free survival | 23 months | 100 months | 60 months | <0.0005 |
| Mean local control time | 31 months | 121 months | 63 months | 0.006 |
| Mean metastasis free survival | 30 months | 165 months | 83 months | <0.005 |
When comparing spinal poorly differentiated chordoma with spinal conventional and chondroid chordoma.
The current case shows the classic histomorphological and immunohistochemical aspects of poorly differentiated chordoma that were described in the literature. The negative S100 staining is common in poorly differentiated chordoma in contrast with conventional chordoma which shows more frequent S100 staining.2 A few peculiar features are present in this case. First, the presence of the non-necrotizing granulomas in the lung and the lymph node was not clearly explained. We favor that those are probably not related to the histologic spectrum of poorly-differentiated chordoma and may represent a separate pathologic process or less likely a reaction to the metastatic tumor. Second, the history of papillary thyroid carcinoma and the age of the patient made the diagnosis more challenging given the need to exclude metastatic papillary carcinoma, possibly undergoing higher grade transformation especially to poorly differentiated or anaplastic carcinoma. The age of the patient which is above the typical age of occurrence for poorly differentiated chordoma further added to the initial diagnostic thought that the tumor was more probably metastatic papillary carcinoma rather than representing a new diagnosis. Furthermore, the rare association of poorly differentiated chordoma with other malignancies can negatively impact expanding the differential diagnosis in similar cases especially in the setting of a widely metastatic tumor that is strongly positive for keratin. In fact, to the best of our knowledge, we believe that this is the first reportable case of poorly differentiated chordoma occurring in association with other malignancies. Whether the association between those two tumors in a short period of time has a specific etiologic link needs further investigations.
Poorly differentiated chordoma has distinct clinical features, immunohistochemical staining profile and prognostic implications. We reported a case of metastatic poorly differentiated chordoma in an adult man with a history of papillary thyroid carcinoma. These tumors should always be considered in the clinical setting of metastatic disease with an axial located mass. The poorly differentiated morphology along with the absence of the typical myxoid matrix and the physaliferous cells poses diagnostic challenges. However, the use of INI-1 and brachyury immunostains is always recommended in such cases. Recognition of this tumor is important as it carries worse prognosis and needs more aggressive therapeutic approaches.
ACKNOWLEDGEMENTS
We gratefully thank Dr. Christopher Fletcher for graciously providing the brachyury immunohistochemical stain slide and photomicrograph.
Footnotes
How to cite: Jaber OI, Ashhab MA. Metastatic poorly differentiated chordoma: the eyes do not see what the mind does not know. Autops Case Rep [Internet]. 2019 Oct-Dec;9(4):e2019120. https://doi.org/10.4322/acr.2019.120
The manuscript was approved by the Institutional review board at King Hussein Cancer Center and is in accordance with the Helsinki Declaration as revised in 2013.
Financial support: None
REFERENCES
- 1.Coffin CM, Swanson PE, Wick MR, Dehner LP. Chordoma in childhood and adolescence: a clinicopathologic analysis of 12 cases. Arch Pathol Lab Med. 1993;117(9):927-33. [PubMed] [Google Scholar]
- 2.Shih AR, Cote GM, Chebib I, et al. . Clinicopathologic characteristics of poorly differentiated chordoma. Mod Pathol. 2018;31(8):1237-45. 10.1038/s41379-018-0002-1. [DOI] [PubMed] [Google Scholar]
- 3.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12(1):1-11. 10.1023/A:1008947301735. [DOI] [PubMed] [Google Scholar]
- 4.Cha YJ, Hong CK, Kim DS, Lee SK, Park HJ, Kim SH. Poorly differentiated chordoma with loss of SMARCB1/INI1 expression in pediatric patients: a report of two cases and review of the literature. Neuropathology. 2018;38(1):47-53. 10.1111/neup.12407. [DOI] [PubMed] [Google Scholar]
- 5.Hasselblatt M, Thomas C, Hovestadt V, et al. . Poorly differentiated chordoma with SMARCB1/INI1 loss: a distinct molecular entity with dismal prognosis. Acta Neuropathol. 2016;132(1):149-51. 10.1007/s00401-016-1574-9. [DOI] [PubMed] [Google Scholar]
- 6.Mobley BC, McKenney JK, Bangs CD, et al. . Loss of SMARCB1/INI1 expression in poorly differentiated chordomas. Acta Neuropathol. 2010;120(6):745-53. 10.1007/s00401-010-0767-x. [DOI] [PubMed] [Google Scholar]
- 7.Hoch BL, Nielsen GP, Liebsch NJ, Rosenberg AE. Base of skull chordomas in children and adolescents: a clinicopathologic study of 73 cases. Am J Surg Pathol. 2006;30(7):811-8. 10.1097/01.pas.0000209828.39477.ab. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CD, Bridge JA, Hogendoorn PC, Mertens F, editors. WHO classification of tumors of soft tissue and bone. Lyon: IARC; 2013. [Google Scholar]
- 9.Hornick JL. Practical soft tissue pathology: a diagnostic approach. Philadelphia: Elsevier/Saunders; 2013. [Google Scholar]
- 10.Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol. 2007;31(12):1813-24. 10.1097/PAS.0b013e31805f6775. [DOI] [PubMed] [Google Scholar]
- 11.Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33(4):542-50. 10.1097/PAS.0b013e3181882c54. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21(6):647-52. 10.1038/modpathol.2008.44. [DOI] [PubMed] [Google Scholar]
- 13.Donner LR, Wainwright LM, Zhang F, Biegel JA. Mutation of the INI1 gene in composite rhabdoid tumor of the endometrium. Hum Pathol. 2007;38(6):935-9. 10.1016/j.humpath.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]