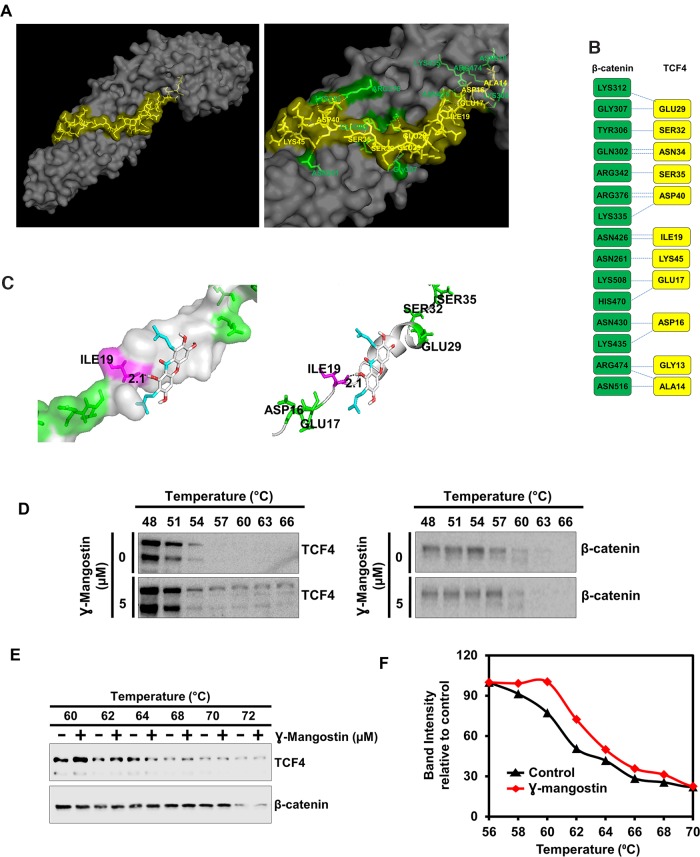
Figure 5. γ-Mangostin interacts with the β-catenin/TCF4 complex.
(A) Left panel shows structure of β-catenin/TCF4 complex (PDB code: 1JDH) and right panel shows the amino acid interactions of β-catenin (Green) and TCF4 (Yellow) structure. (B) Protein-protein interface of β-catenin (Green) and TCF4 (Yellow) structure. Residue interactions by hydrogen bonds (blue dotted line) across interface were shown. (C) Molecular docking study shows γ-Mangostin binds to TCF4 protein in beta-catenin binding site (Left panel: Cartoon view, right panel: surface view). γ-Mangostin interacts with Ile-19 (2.1 Å) of TCF4 with the binding energy of -5.5 Kcal/mol. (D) Cellular thermal shift assay (CETSA)-Method I. HCT116 cells were treated with γ-Mangostin (5 µM) and subjected to differential temperature treatment for 3 mins. Resulting lysates were subjected to western blot analysis. γ-Mangostin protected TCF4 against thermal degradation, suggesting that the compound interacts with the receptor. (E-F) Cellular thermal shift assay (CETSA)-Method II. HCT116 cell lysates were collected and treated with γ-Mangostin (20 µM) and subjected to differential temperature treatment for 3 mins. Resulting lysates were subjected to western blot analysis. γ-Mangostin protected TCF4 at 60 ºC and 62ºC against thermal degradation, again suggesting that the compound interacts with the receptor.