Figure 1.
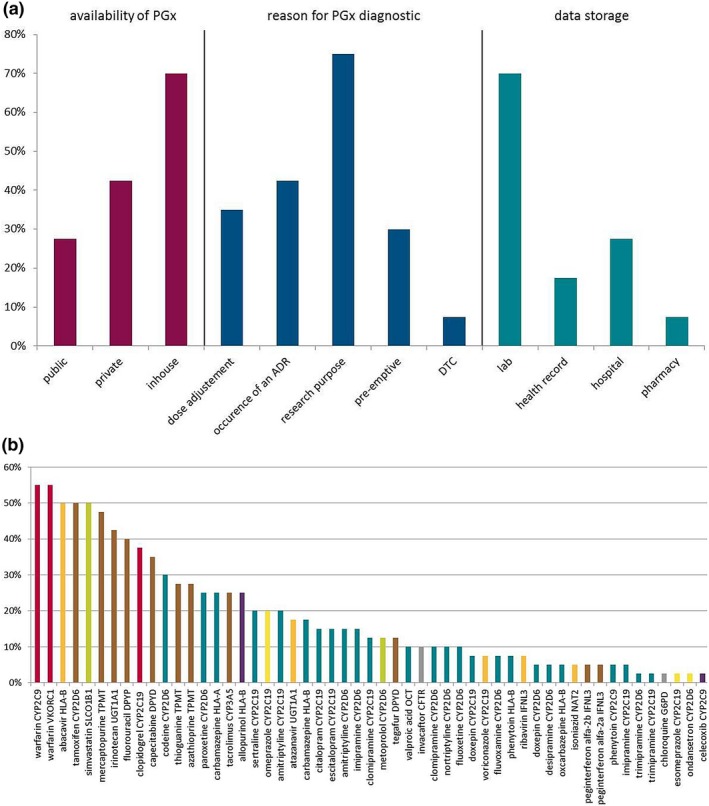
Use and availability of pharmacogenetic tests in clinical practice in Europe: data from the survey done in the Ubiquitous Pharmacogenomics project. (a) Answers to a survey concerning availability of pharmacogenomics (PGx) test (red), reason for ordering a PGx test (blue), and PGx test data storage (turquoise). Participants were able to choose more than one answer. Public: health insurances, public health system; private: companies; in‐house: hospital, laboratory; DTC: direct‐to‐consumer. (b) Importance of drug–gene pairs as rated by participants. Percentages of participants ranking the 10 most important drug–gene pairs from a list of 56 pairs of drug–gene pairs available as Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines are shown. The color of each bar indicates the class of the drug in the drug–gene pairs: red: antithrombotics; orange: antiinfectives; brown: antineoplastic and immunomodulating agents; turquoise: drugs acting on the central nervous system; green: drugs acting on the cardiovascular system; purple: drugs acting on the musculoskeletal system; yellow: drugs acting on the alimentary tract and metabolism; gray: other drugs like antiparasitic products and drugs acting on the respiratory system. ADR, adverse drug reaction; CFTR, cystic fibrosis transmembrane conductance regulator; CYP, cytochrome P450; DPYD, dihydropyrimidine dehydrogenase; HLA, human leukocyte antigen; IFNL3, interleukin 28B; NAT2, N‐acetyltransferase 2; OCT, organic cation transporter; SLCO1B1, solute carrier organic anion transporter family member 1B1; TPMT, thiopurine methyltransferase; UGT1A1, uridine diphosphate glucuronosyltransferase; VKORC1, vitamin K epoxide reductase complex subunit 1. Responder rate: n = 40 (82%, total: 49 participants from eight different countries (United Kingdom, the Netherlands, Germany, Slovenia, Greece, Italy, Portugal, and Canada).
