Figure 2.
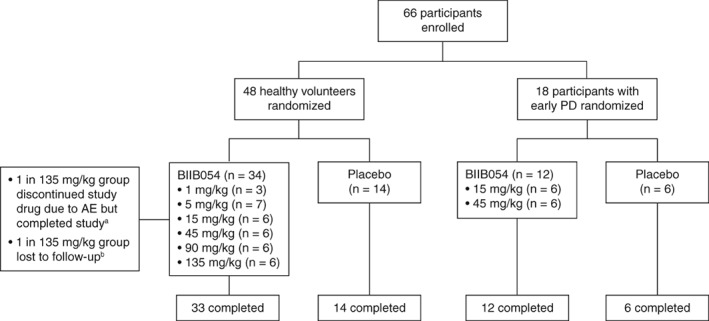
Patient disposition. aParticipant received 32% of BIIB054 dose because of grade 1 hypersensitivity reaction but completed study. bParticipant could not be contacted after the week 3 visit despite multiple phone calls and certified letters, was listed as lost to follow‐up, and did not complete the study. AE, adverse event; PD, Parkinson's disease.
