Table 1.
Optimization of the reaction conditions.[a]
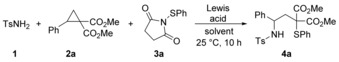
| |||||
|---|---|---|---|---|---|
| Entry | Lewis acid | [mol %] | 3 a [equiv] | Solvent | Yield [%] |
| 1 | AlCl3 | 20 | 1.3 | DCE | 0 |
| 2 | MgI2 | 20 | 1.3 | DCE | 0 |
| 3 | Sc(OTf)3 | 20 | 1.3 | DCE | 0 |
| 4 | Sn(OTf)2 | 20 | 1.3 | DCE | 69 |
| 5 | Y(OTf)3 | 20 | 1.3 | DCE | 0 |
| 6 | Sn(OTf)2 | 5 | 1.3 | DCE | 0 |
| 7 | Sn(OTf)2 | 10 | 1.3 | DCE | 86 |
| 8 | Sn(OTf)2 | 10 | 1.3 | CH2Cl2 | 29 |
| 9 | Sn(OTf)2 | 10 | 1.3 | dioxane | 0 |
| 10 | Sn(OTf)2 | 10 | 1.7 | DCE | 93 |
| 11 | Sn(OTf)2 | 10 | 2.3 | DCE | 72 |
| 12[b] | Sn(OTf)2 | 10 | 1.3 | DCE | 35 |
[a] Reaction conditions: 1 (165 μmol), Lewis acid, solvent (1.5 mL), 2 a (150 μmol), 3 a, 25 °C, 10 h, Ar atmosphere; yields represent isolated and purified products; [b] The corresponding phthalimide derivative was used instead of 3 a.
