Figure 3.
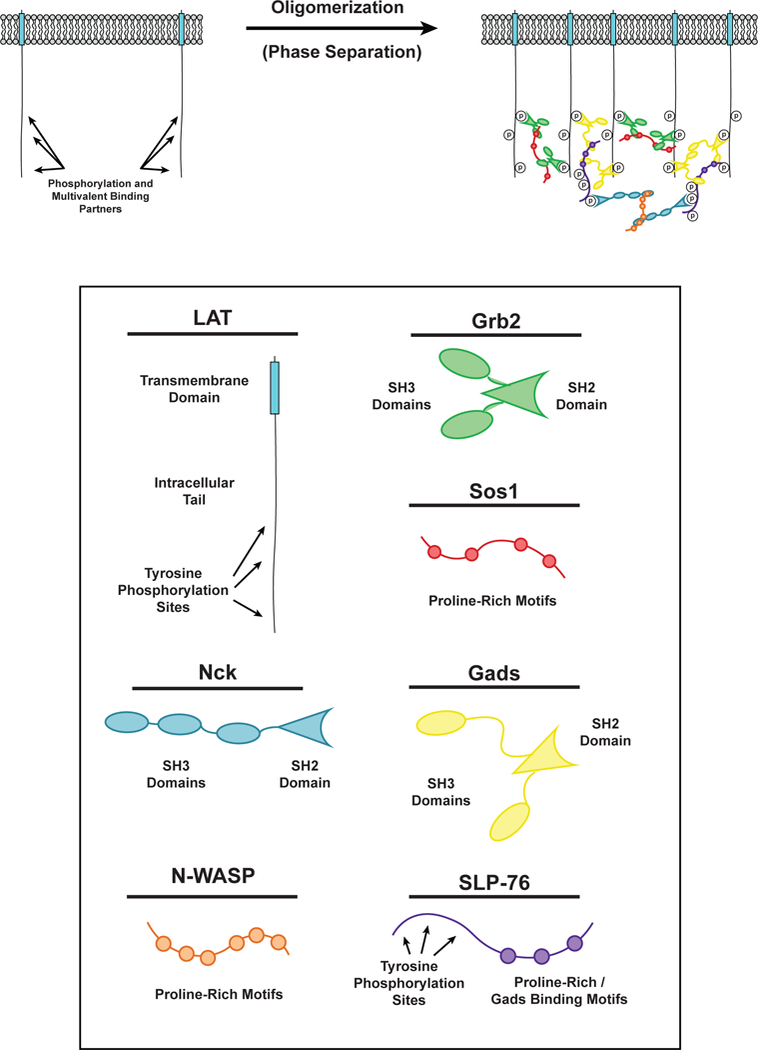
Phase separation of LAT in T cell signaling. (LEFT) LAT transmembrane adaptor proteins can be phosphorylated at three tyrosine residues in the disordered tail to created binding sites for the multivalent adaptor proteins Grb2 (Green) and Gads (Yellow). (RIGHT) Phosphorylated tyrosine residues on LAT can be bound by the SH2 domain of Grb2. The SH3 domains of Grb2 bind to proline-rich motifs of Sos1 (Red) to form a phase-separated clusters of LAT, Grb2, and Sos1. Similarly, phosphorylated tyrosine residues on LAT can be bound by the SH2 domain of Gads. The SH3 domains of Gads bind to proline-rich motifs and a RxxK motif in SLP-76 (Purple) to form a phase-separated cluster of LAT, Gads, and SLP-76. Phosphorylated tyrosine residues on SLP-76 can be bound by the SH2 domain of Nck (Cyan). The SH3 domains of Nck bind to the proline-rich motifs of N-WASP (Orange) to create another level of multivalent interactions that can contribute to the phase separation of LAT on the membrane of T cells.
