Figure 7.
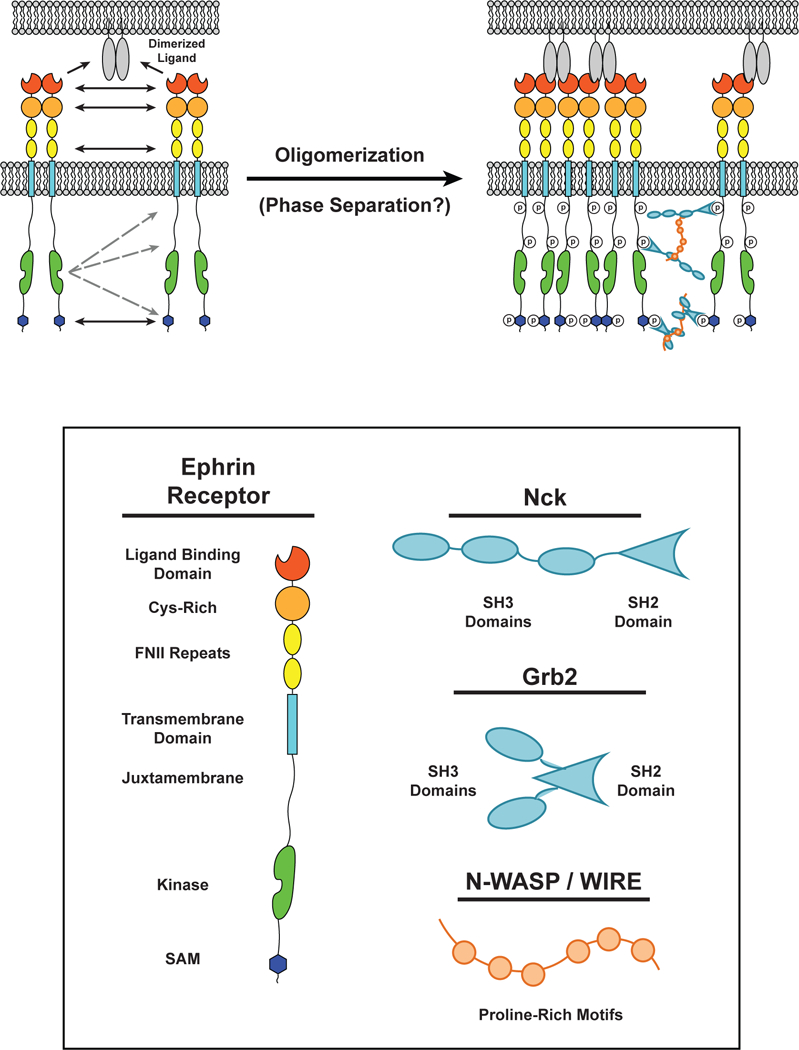
Multiple interactions drive dimerization and cluster formation of ephrin receptors. (LEFT) Interactions between two ephrin receptor EC ligand binding domains (LBDs), Cys-rich domains, and fibronectin-like (FN) domains can result in ephrin receptor dimerization in the absence of ligands. Dimerized ligands expressed on the surface of an apposing cell can seed cluster formation. (RIGHT) Upon binding to a ligand, the kinase domain of one ephrin receptor can transphosphorylate intracellular tyrosine residues on surrounding receptors. These residues can serve as docking sites for Src Homolgy (SH) SH2 / SH3 adaptor proteins, such as Grb2 and Nck, which can oligomerize through multivalent interactions with proteins containing Proline-Rich Motifs (PRMs), such as N-WASP. The intracellular Sterile Alpha Motifs (SAMs) also self-associate to promote receptor oligomerization.
