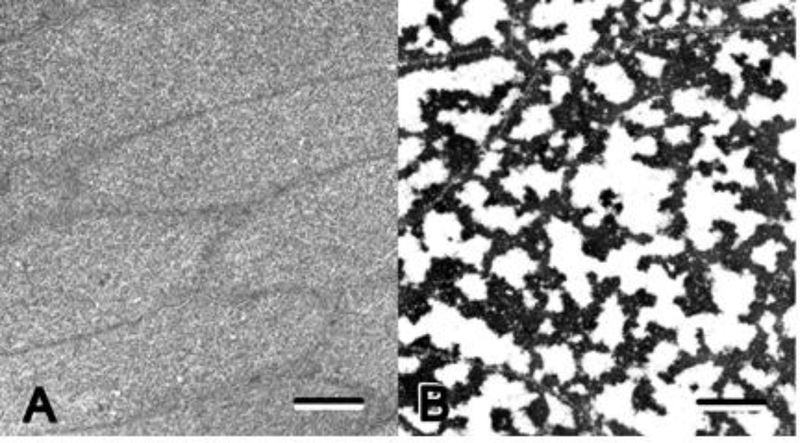
FIGURE 2: Cellular Opacification (cataract) in Mouse is an Excellent Model for the Study of Protein Aggregation and Amyloid Formation in vivo.
Transmission electron micrographs of transparent (left) and opaque (right) lens cells illustrate differences between cytoplasmic structure in the normal transparent lens and a dense cataract. In the transparent cells, soluble, concentrated proteins are distributed uniformly throughout the cells and fluctuations in the index of refraction are small relative to the wavelength of visible light (390–700nm). The distribution of the cytoplasmic proteins is uniform and homogeneous similar to window glass. In opaque cells, the cytoplasmic proteins self-assemble into condensed, light scattering aggregates separated by spaces filled with cytoplasmic fluid. The fluctuations in the index of refraction are large relative to the wavelength of visible light similar to frosted glass. In this example the condensed protein appears to have a filamentous structure connecting the cell membranes, but various morphologies are observed in opacities, and they can be very subtle [47–50]. A lens containing opaque cells like those in figure 2B would appear completely opaque as in the cataract in figure 3. It is well known, but not widely recognized, that only 3 percent of the cytoplasmic proteins need to self- assemble into HMW for complete cellular opacity. Regulation of the interactions between cytoplasmic proteins is a function of alphaB crystallin that is thought to assist in stabilization of transparent cytoplasm both during the differentiation of transparency and during aging when post-translational modification and proteolysis can favor aggregation and disordered cytoplasmic structure. (bar = one micron)