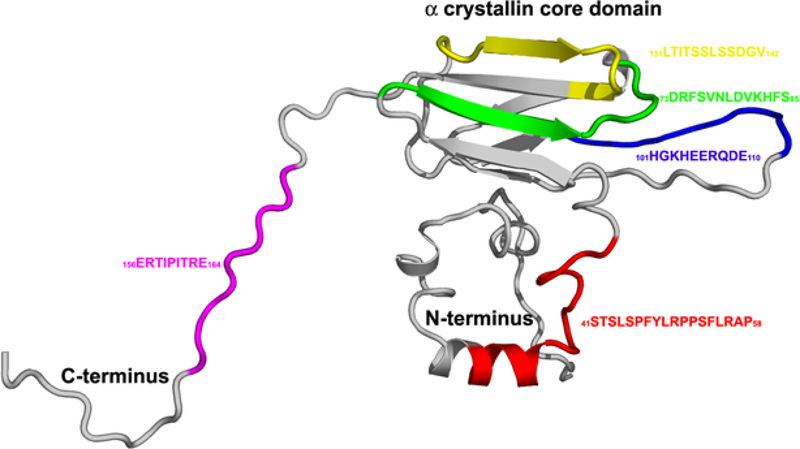
FIGURE 5: Model for the interactive sequences in a ribbon model of the small heat shock protein, human alphaB crystallin.
Possibly the most surprising result of the study of the interactive sequences in human alphaB crystallin was the identification of multiple interactive sequences. The sequences form a diverse network of potential protective sites for selective interaction with several categories of target proteins. The biological, biophysical and pharmacological advantages of combining a number of multifunctional interactive sites on a single intracellular protective molecule, instead of numerous soluble cytoplasmic peptides, as small as four amino acids, each with protective activity remains to be determined.