Figure 2.
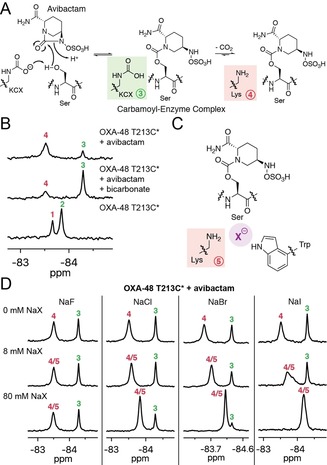
19F NMR studies on the interaction of OXA‐48 T213C* with avibactam and halide ions. (A) Proposed interaction of avibactam with a class D serine β‐lactamase, showing the carbamylated (green) and uncarbamylated (red) states of the complex. (B) 19F NMR spectra showing the impact of avibactam on the carbamylation state of OXA‐48 T213C* with and without added sodium bicarbonate. (C) Proposed binding mode of a halide ion in the uncarbamylated active site of the OXA‐48:avibactam complex, based on previous crystallographic work.5 (D) 19F NMR spectra showing the impact of halide ions on the carbamylation state and 19F chemical shifts of OXA‐48 T213C*. Note that the addition of sodium fluoride may also affect the pH of the sample. Trifluoroethanol was used as an internal standard for 19F NMR, and CFCl3 in CDCl3 was used as an external standard.
