Figure 2.
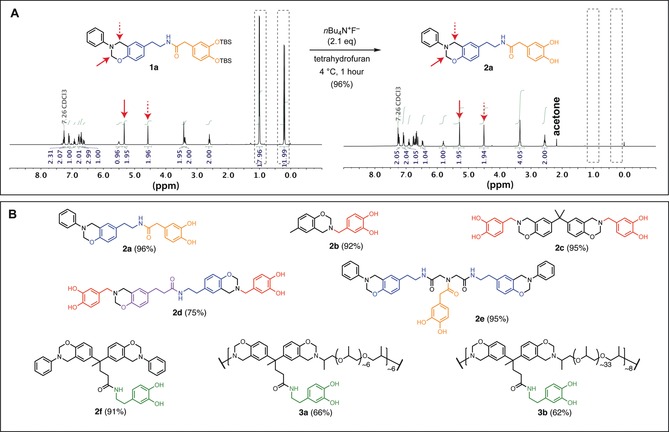
Protecting group strategy used to prepare bioinspired benzoxazine monomers. A) TBS‐protected benzoxazine monomers are readily deprotected with TBAF to provide catechol‐modified benzoxazine monomers, demonstrated in the conversion of 1 a to 2 a. Peaks corresponding to TBS groups disappear after deprotection (grey dashed boxes), and characteristic benzoxazine resonances are present prior to and following deprotection in 1H‐NMR spectra (red arrows). B) This strategy was applied to access a family of bioinspired benzoxazines from biological precursors; yields of the deprotection reactions are shown in parentheses. Colors correspond to the naturally occurring building blocks used to prepare the highlighted fragment, which have the potential to be microbially sourced in the future. (red: 3,4‐dihydroxybenzaldehyde; orange: 3,4‐dihydroxyphenylacetic acid; green: dopamine; blue: tyramine; violet: phloretic acid).
