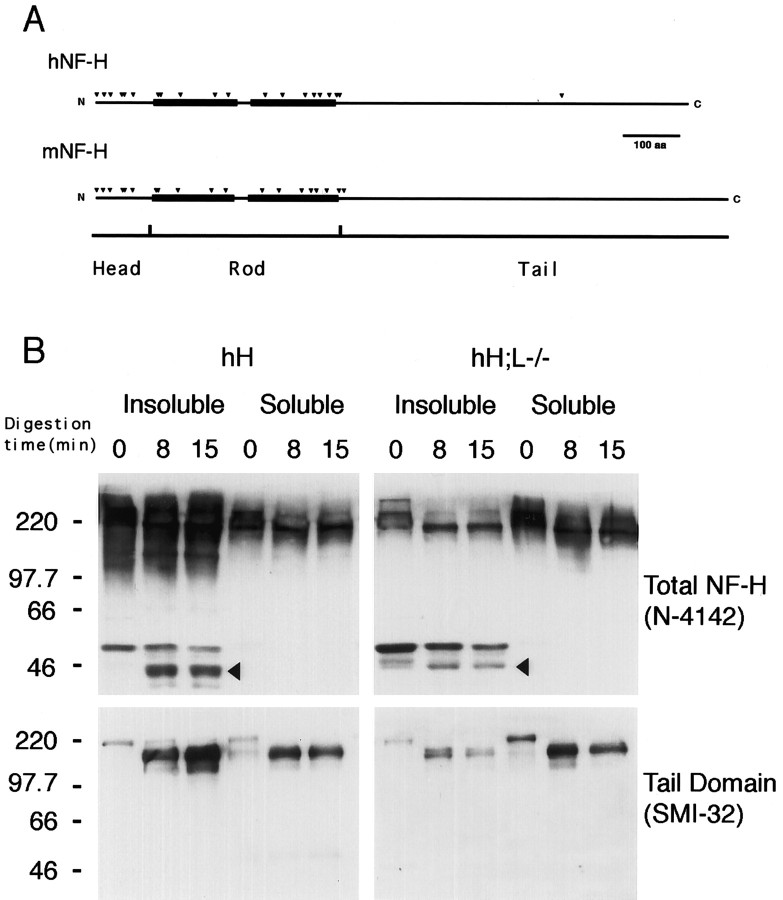
Fig. 5.
Partial α-chymotrypsin digestion of insoluble NF-H. A, Potential α-chymotrypsin digestion sites (black arrowheads) along the head and rod domains of both mouse (mNF-H) and human (hNF-H) NF-H proteins. Black boxesindicate the α-helical segments of NF-H rod domain. B, Western blot analysis of the α-chymotrypsin digestion products. Soluble and insoluble fractions from spinal cord homogenates of hNF-H transgenic or hH;L−/− mice were treated with α-chymotrypsin for 0, 8, and 15 min as described in Materials and Methods. The immunodetection of NF-H digestion fragments was performed using an anti-NF-H polyclonal antibody (N-4147, 1:2000 dilution) or the Smi-32 (1:500 dilution) monoclonal antibody, which is specific to unphosphorylated (Lys/Ser/Pro) residues present in the tail domains of both human and mouse NF-H proteins. Digestion of soluble NF-H proteins from both mouse models led to the formation of a single large fragment immunoreactive for both Smi-32 and NF-H polyclonal antibody, whereas digestion of insoluble NF-H proteins led to the formation of additional NF-H fragments (black arrowheads) that were only immunoreactive with the polyclonal antibody. Numbers on the left of gels in B correspond to molecular weight markers in kilodaltons.